October 6, 2009 - Boston Scientific Corp. announced it has received 510(k) clearance from the U.S. Food and Drug ...
Stents Bare Metal
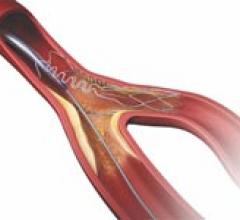
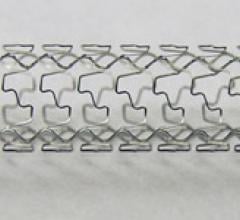
Bare metal stents are mesh-like tubes of thin wire without a coating or covering. Ideally, bare metal stents will be covered by a new layer of endothelial cells, sealing it into the vessel wall, within a few weeks after implant. However, a percentage of patients have their body negatively respond to the implanted foreign body, resulting in the formation of neointimal hyperplasia, a growth of scar tissue over the stent. This can cause restenosis of the vessel segment, which led to the development of drug-eluting stents (DES) now used in most patients. However, DES require the patient to take long-term antiplatelet medication, so patients who are likely to be noncompliant are often given bare metal stents.
September 29, 2009 – Boston Scientific Corp. Sept. 25 announced two-year follow-up data from the HORIZONS-AMI ...

Bare metal stents (BMS) were introduced in 1994 as an improvement over balloon angioplasty alone, which had a high ...
September 15, 2009 – Tryton Medical Inc. said today its Tryton Side Branch Stent has been used in 250 procedures ...
September 14, 2009 – OrbusNeich's Genous Bio-engineered R stent is feasible and safe in patients who need ...

Gregg W. Stone, M.D., offered a preview Sept. 10 of some of the key, late-breaking clinical trials and trends in ...
August 24, 2009 – Prescient Medical Inc. said today it received European CE mark clearance to commercialize its ...
July 9, 2009 – Coherex Medical Inc. today said its Coherex FlatStent EF PFO Closure System has been granted CE ...
June 30, 2009 - Stentys this week said it secured the second tranche of its series B financing, which will enable ...
June 24, 2009 – CorNova Inc. said this week it received CE mark approval for its Valecor Platinum Coronary Stent ...
June 8, 2009 - The Bypass Angioplasty Revascularization Investigation With Diabetes Trial (BARI-2D) is a useful ...
June 1, 2009 - Six months after placement of a vProtect Luminal Shield to treat a "vulnerable plaque,” a follow ...
May 21, 2009 - InspireMD Ltd. said today it completed enrollment for the MAGICAL Trial (MGuard in Acute ...
April 1, 2009 - Medtronic Inc. this week began the international launch of the Driver Sprint RX Coronary Stent ...
March 30, 2009 - An AHRQ-funded study finds a lower risk of death and heart attack in heart disease patients 65 ...


 October 06, 2009
October 06, 2009