September 24, 2010 - Two-year data from the SPIRIT IV trial show that everolimus-eluting stents demonstrated ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
September 23, 2010 - Results from the second stage of a trial studying a bioresorbable vascular scaffold were ...
September 23, 2010 - Results from a study evaluating the safety and clinical efficacy of the Promus Element ...
September 22, 2010 – Coronary artery disease, a leading cause of death and poor quality of life worldwide, can ...
September 21, 2010 – A new drug-eluting stent system has received CE mark approval for diabetic patients and those ...
September 20, 2010 – Patient enrollment has started in the EXCELLA BD Randomized Clinical Trial designed to ...
September 14, 2010 – The latest clinical trial data for the Promus Element and Taxus Element coronary stents will ...
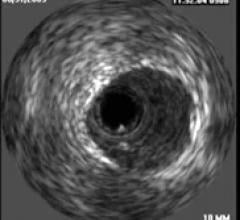
September 8. 2010 – Intravascular ultrasound (IVUS)-guided bifurcation stenting significantly reduced the four ...
September 7, 2010 – New analyses of subgroups from the SORT OUT III study provide detail on longer-term follow-up ...
September 1, 2010 - The results of follow-up tests undertaken ten years after the first patient was treated with ...
September 1, 2010 - A new zotarolimus-eluting coronary stent using a biocompatible polymer gained CE mark and is ...

In the world of endovascular devices, the rule is no longer one size fits all. The recent increase in the variety ...
August 24, 2010 – Optical coherence tomography (OCT) evaluation of a patient with anterior ST-elevation ...
August 19, 2010 – The Cardiovascular Research Foundation recently announced the late-breaking trials and first ...
August 3, 2010 — Patient enrollment began in the EVOLVE clinical trial, which is designed to assess the safety and ...


 September 24, 2010
September 24, 2010