March 8, 2011 – The U.S. Food and Drug Administration (FDA) announced monthly liver enzyme tests are no longer ...
Vascular Access
This channel includes news and new technology innovations for vascular access, both venous and arterial access, for percutaneous, transcatheter, interventional devices. In cath lab procedures, an introducer sheath needs to be inserted into the vessel to allow access of guidewires, catheters and devices into the body. Introducer sheaths are self-sealing to serve a stop cock to prevent blood from leaving the body during procedures. There are various access sites that can be accessed, most commonly femoral or radial access, but also the ulnar, axillary and tibiopedal vasciular access routes There are several complications that can occur at the access site and technologies have been developed to reduce these issues, especially with the most common bleeding complications.
February 9, 2011 – The U.S. Food and Drug Administration (FDA) said certain lots of the Arstasis One femoral ...
February 8, 2011 – The U.S. Food and Drug Administration (FDA) said Merit Medical Systems initiated a Class I ...
January 27, 2010 – An ergonomic, peel-away introducer sheath was launched this week to facilitate placement of ...
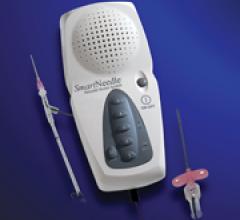
January 11, 2011 – A new vascular access system is available in the United States and Europe. The SmartNeedle ...
December 21, 2010 – A new introducer kit is now available in the United States. The VSI StraitSet Kit, from ...
November 16, 2010 – An all-in-one micropuncture radial access kit is now available in the United States. The ...
September 27, 2010 - Treating varicose veins with a new access kit reduces the number of steps and reduces ...
August 17, 2010 – Certain batches of the St. Jude Medical 6 French Engage Introducer are being recalled because ...
August 11, 2010 – New sizes of the SoloPath TransFemoral Endovascular Access Catheter were recently cleared by ...
March 11, 2010 – The FDA cleared a new family of introducers which offer features intended to minimize trauma to ...
February 3, 2010 – The new Gator ClipSeal Plug is designed to maintain a hemostatic seal around 0.035- or 0.038 ...
February 1, 2010 – The FDA granted market clearance for the PICC WAND introducer catheter, which enables ...
December 31, 2009 – A new micro-introducer kit launched this week features a stiffened introducer option. The ...
November 17, 2009 – Following three reports of stretching and fractures during procedures, the ViperSheath Sheath ...


 March 07, 2011
March 07, 2011