March 7, 2024 — BioCardia, Inc. , a biotechnology company focused on advancing late-stage cell therapy interventions for cardiovascular disorders, today announced positive interim results from the Phase III randomized controlled trial of its CardiAMP autologous cell therapy in 110 randomized patients with advanced chronic heart failure at a mean 20-month follow-up, (CardiAMP HF). Results showed reductions in heart death equivalents and MACCE, with a magnified reduction among patients with elevated NTproBNP, a common marker of heart distress. The data was presented today by Amish Raval, MD, Director of Clinical Cardiovascular Research and Professor of Medicine at the University of Wisconsin-Madison at the Technology and Heart Failure Therapeutics (THT) 2024 annual meeting.
Over a mean 20 months of follow-up, patients with advanced chronic heart failure who received a single endomyocardial dose of autologous CardiAMP cell therapy while on maximal medical therapy had a 37% relative risk reduction in all-cause heart death equivalents and a 9% relative risk reduction in non-fatal incidence of heart attacks, strokes, and hospitalization due to heart failure (MACCE). Patients treated with CardiAMP cell therapy saw an almost 5% lower rate of heart death equivalents at up to two years compared to control patients treated with heart failure medication alone (8.3% vs. 13.2%, respectively). CardiAMP cell therapy was also associated with trends toward reduced ventricular tachyarrhythmias, enhanced heart function as measured by left ventricular ejection fraction, and improved NTproBNP.
In a subgroup analysis of patients with elevated NTproBNP at baseline – encompassing 59% of total enrolled randomized patients – patients treated with CardiAMP cell therapy experienced an 86.2% relative risk reduction in heart death equivalents and a 23.9% relative risk reduction in MACCE. These patients saw more than a 17% lower rate of heart death equivalents at up to two years compared to control patients treated with heart failure medication alone (2.9% vs. 21.1%, respectively).
“These positive results for CardiAMP cell therapy are very encouraging, especially for patients with elevated NTproBNP, who encompass the majority of heart failure patients that we see in our daily practice,” said trial co-principal investigator Dr. Raval. “While the trial’s data safety monitoring board determined that the study would not meet its composite primary endpoint that included six-minute walk distance per the trial design, the positive results for reduced heart death equivalents, reduced MACCE, and safety indicate potential for this therapy to improve outcomes for patients with advanced chronic heart failure. Despite improvements with current medications and devices, heart failure remains at epidemic proportions and we now have an exciting opportunity for a therapy to improve important, objective outcomes, such as mortality and hospital re-admissions rates. I am excited to be part of a terrific team to validate the promise of this therapy in this high responder group in the follow-on trial that is now FDA-approved and soon to treat its first patient.”
“We thank the FDA for its speedy review and approval of the important follow-on Phase III trial. We are encouraged by the totality of today’s results and anticipate that both the final 24-month data analysis and follow-on trial outcomes would be consistent with this highly positive data,” said BioCardia CEO Peter Altman.
Also at the THT scientific meeting, first enrollment for the dose escalation safety phase of BioCardia’s Phase I/II study of the CardiALLO™ allogeneic mesenchymal stem cell (MSC) therapy in heart failure patients was reported. The cohort receiving the lowest dose of 20 million cells has been initiated with no treatment-emergent adverse events, arrhythmias, rejection, or allergic response. The first author of the study is R. David Anderson, MD, Professor of Medicine at the University of Florida at Gainesville. Following completion of the dose escalation safety phase of the study, a Phase II randomized double-blind controlled study is planned to assess efficacy.
About BioCardia’s CardiAMP Autologous Cell Therapy Program*
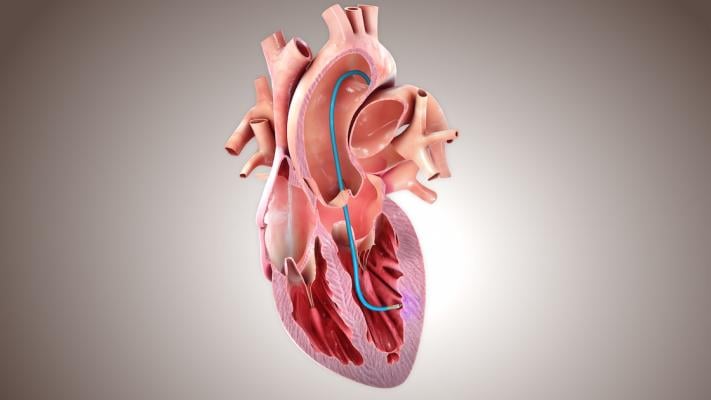
Designated by the FDA as a Breakthrough Therapy, CardiAMP Cell Therapy uses a patient’s own (autologous) bone marrow cells delivered to the heart in a minimally invasive, catheter-based procedure to potentially stimulate the body’s natural healing response. CardiAMP Cell Therapy incorporates three proprietary elements not previously utilized in investigational cardiac cell therapy: a pre-procedural cell analysis for patient selection, a high target dosage of cells, and a proprietary delivery system that has been shown to be safer than other intramyocardial delivery systems and exponentially more successful in cell retention. The CardiAMP HF trial is supported by the Maryland Stem Cell Research Fund and has reimbursement from the Centers for Medicare and Medicaid Services (CMS) for both treatment and control procedures. The CardiAMP HF II trial is expected to similarly secure CMS reimbursement.
About BioCardia’s CardiALLO Allogeneic Cell Therapy Program
CardiALLO allogeneic cell therapy provides an “off the shelf” mesenchymal stem cell therapy typically derived from younger donors. These cells are immunomodulatory, with the potential to impact inflammatory processes in heart failure and have been shown to release multiple critical angiogenic factors that can enhance microvascular function and capillary networks in ischemic tissue. CardiALLO therapy for heart failure uses BioCardia’s new MSC manufacturing process, which builds on the experience of three completed co-sponsored MSC clinical trials encompassing 84 treated patients in the same indication with the same delivery platform.
For more information: https://www.biocardia.com/
Related content:
Is Cardiac Cell Therapy Worth Another Look?


 February 03, 2026
February 03, 2026 









