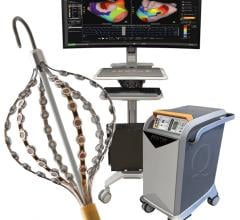
January 13, 2011 – A new robotic navigation system has both received the CE mark and undergone its first successful cardiac ablation procedure. The Vdrive robotic navigation system, from Stereotaxis, provides a companion to magnetic navigation.
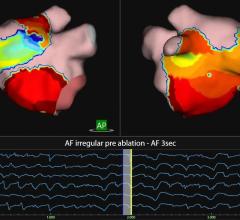
In the first procedure with the system, Tamas Szili-Torok, M.D., Ph.D., from the department of clinical electrophysiology at the Erasmus Medical Center in The Netherlands, successfully treated atrial fibrillation in a patient with known difficult heart anatomy. Szili-Torok was able to complete the entire case without having to manually adjust the circular mapping catheter.
"This first use of the Vdrive robotic navigation system exceeded my expectations," Szili-Torok said. "The robotic navigation of the circular mapping catheter was intuitive and allowed me to make multiple small precise adjustments from the control room to efficiently treat my patient's atrial fibrillation."
Stereotaxis' system is a robotic navigation technology that complements the company’s Remote Magnetic Navigation System. Designed to manipulate accessory devices such as variable loop catheters, steerable sheaths and ultrasound catheters, it combines magnetic navigation with the Odyssey information management system to bring precise control during catheter-based electrophysiology procedures.
The Vdrive system is available in the European Union. It is not currently available for purchase in the United States as it is still under 510(k) review by the U.S. Food and Drug Administration.
For more information: www.stereotaxis.com, www.odysseyexperience.com


 September 05, 2025
September 05, 2025