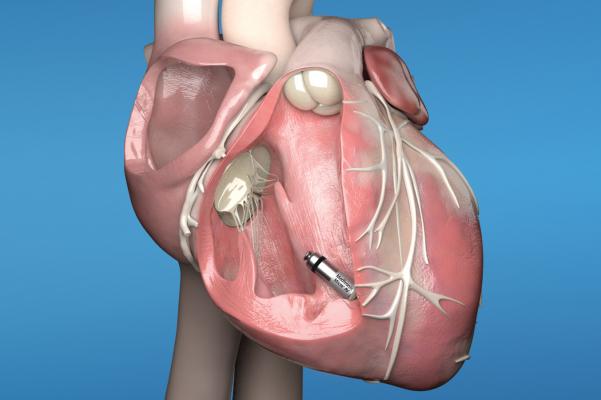
February 13, 2020 — The U.S. Food and Drug Administration (FDA) has approval of Micra AV, the world’s smallest pacemaker with atrioventricular (AV) synchrony. The leadless Micra AV device is indicated for the treatment of patients with AV block, a condition in which the electrical signals between the chambers of the heart (the atria and the ventricle) are impaired.
Medtronic said it now offers the first FDA-approved leadless pacemaker portfolio, expanding the number of potential candidates for this groundbreaking technology in the U.S.
“With the approval of Micra AV, more pacemaker patients qualify for a new treatment option that offers the advantages of leadless pacing – including a minimally invasive implant procedure and a cosmetically invisible device,” said Larry Chinitz, M.D., cardiac electrophysiologist and director of NYU Langone’s Heart Rhythm Center in New York City. “Although complications with traditional pacemakers are infrequent, when they occur, they’re expensive to treat and can be invasive for the patient. Real-world use of Micra has shown a 63% reduction in major complications compared to traditional pacemakers.
Historically, patients with AV block have been treated with traditional dual-chamber pacemakers which are implanted in the upper chest, under the skin below the collar bone, and connected to the heart using thin wires called “leads.” Identical in size and shape to the original Micra Transcatheter Pacing System (TPS), Micra AV has several additional internal atrial sensing algorithms which detect cardiac movement, allowing the device to adjust pacing in the ventricle to coordinate with the atrium, providing “AV synchronous” pacing therapy to patients with AV block.

MARVEL 2 Data Supported FDA Clearance Mica AV to Coordinate Pacing Between the Atrium and Ventricle
The Micra AV approval is based on data from the MARVEL 2 (Micra Atrial Tracking Using A Ventricular accELerometer) study, which evaluated the safety and effectiveness of accelerometer-based atrial sensing algorithms. The study evaluated the ability of the Micra’s internal sensor to monitor and detect atrial contractions and enable coordinated pacing between the atrium and ventricle, thereby providing AV synchrony. Results from the study, presented at the American Heart Association 2019 Scientific Sessions and published simultaneously in JACC: Clinical Electrophysiology,[1] showed the primary efficacy objective was met, with a significantly greater percentage of complete heart block patients with normal sinus rhythm having >70% AV synchrony during algorithm-mediated AV synchronous pacing (38 of 40 patients, 95%) than VVI pacing (0 patients, P<0.001 for proportion of patients with >70% synchrony). The study’s primary safety objective was also met, with no pauses or episodes of pacing-induced tachycardia reported during algorithm mediated AV synchronous pacing.
Medtronic will begin training field personnel and physicians, and will activate a limited number of implanting centers in the coming weeks, with full launch anticipated later this spring.
About the Micra Transcatheter Pacing System (TPS)
Approved by the FDA in 2016, the Micra TPS is a leadless pacemaker option for patients who only require pacing in the right ventricle. Comparable in size to a large vitamin, Micra is less than one-tenth the size of traditional pacemakers yet delivers advanced pacing technology to patients via a minimally invasive approach. During the implant procedure, the device is attached to the heart with small tines and delivers electrical impulses that pace the heart through an electrode at the end of the device.
Unlike traditional pacemakers, Micra does not require leads or a surgical "pocket" under the skin, so potential sources of complications related to leads and pockets are eliminated - as are any visible signs of the device.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. Medtronic strives to offer products and services of the highest quality that deliver clinical and economic value to healthcare consumers and providers around the world.
View an animation of the Micra AV in use.
For more information: www.medtronic.com
Related Micra Leadless Pacemaker Content:
FDA Clears Medtronic Micra AV to Treat AV Block
VIDEO: Current State of Leadless Pacemaker Technology — Interview with Vivek Reddy, M.D.
VIDEO: How to Implant the Micra Leadless Pacemaker
New Algorithms in Medtronic Micra Pacemaker May Improve Synchrony and Cardiac Function in AV Block
FDA Approves World's Smallest Pacemaker for U.S. Patients
Safety, Performance of the World's Smallest Pacemaker Reinforced in Real-world Patients
One-Year Results for Micra TPS Pacemaker Trial Presented at ESC 2016
Leadless Pacemaker Gains Medicare Reimbursement
Wireless and Leadless Pacemaker Being Developed by Texas Heart Institute With Federal Grant
Reference:


 January 29, 2026
January 29, 2026 









