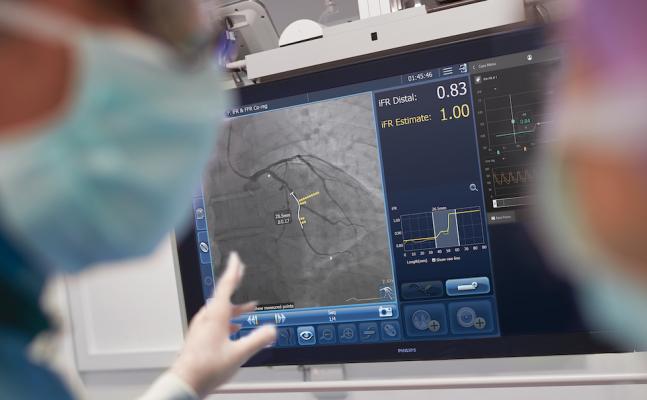
The Philips Healthcare SyncVision system co-registers iFR hemodynamic blood flow measurements to live angiograms to enable easier assessment of which lesions in a vessel need to be stented and which can be left alone and treated medically.
June 22, 2021 – Philips Healthcare announced the official start of the DEFINE GPS study, with the first patient being enrolled by the study’s Principal Investigator Allen Jeremias, M.D., at St Francis Hospital in Roslyn, N.Y. The global, multi-center, prospective, randomized controlled DEFINE GPS study will investigate if guidance by instantaneous wave-free ratio (iFR) measurements co-registered on the angiogram demonstrates superior outcomes and improves the cost-effectiveness of percutaneous coronary intervention (PCI) procedures to open blocked coronary arteries.
DEFINE GPS employs an adaptive study design, estimated to include up to 3,200 participants across 100 sites worldwide and will be one of the largest studies ever sponsored by Philips.
European and U.S. clinical guidelines already endorse the use of physiological measurement of coronary function in PCI procedures, with iFR and fractional flow reserve (FFR) measurements being used to diagnose the significance of a narrowed coronary artery and determine patient selection for treatment. While iFR uses the same pressure guide wires and equipment as FFR, it avoids the use of hyperemic agents (vasodilators) such as adenosine that can adversely affect some patients.
The DEFINE GPS (Distal Evaluation of Functional performance with Intravascular sensors to assess the Narrowing Effect: Guided Physiologic Stenting) study will evaluate the use of iFR measurements in combination with Philips image guided co-registration system, SyncVision, to enhance PCI guidance and provide superior treatment outcomes. DEFINE GPS is a follow-up to the DEFINE PCI study [1] – a one-year trial sponsored by Philips that evaluated the potential of treating residual ischemia in order to improve clinical outcomes for coronary stent patients.
“The DEFINE GPS trial, designed to explore the promising hypotheses that emerged from DEFINE PCI, is a landmark trial that heralds the promise of post-PCI physiologic assessment,” said Gregg W. Stone, M.D., chairman of the DEFINE GPS trial and the director of academic affairs for the Mount Sinai Heart Health System, New York. “While the benefits of physiology prior to PCI are unquestioned, this large-scale trial will definitively demonstrate whether after an angiographically successful PCI procedure the identification and treatment of unsuspected residual ischemia by routine iFR assessment can further improve patient event-free survival.”
“Conducting rigorous clinical science is how we advance patient care, and, like the seminal FAME study that was carried out over 10 years ago, I believe DEFINE GPS has the potential to change the current standard of care in PCI,” said Jeremias. “PCI has made a major positive impact on many coronary artery disease patients’ lives, but when we look back at all the major, high-quality stent trials over the past 20 years we see that around 20-30% of patients continue to have recurring chest pain at one year after receiving treatment. With DEFINE GPS we will be able to definitively determine if a physiology-based PCI approach results in superior patient outcomes compared to standard angioplasty.”
PCI is an image-guided, minimally-invasive treatment to open a coronary artery blockage (stenosis) that is causing a reduced blood flow (ischemia) to heart tissue. Under the current standard of care, clinicians navigate a balloon catheter and coronary stent to the treatment area using interventional X-ray guidance. In the DEFINE GPS study, an iFR pullback measurement, which uses pressure wires to map the physiological profile of disease distribution along the length of the affected vessel, will be overlaid on the angiogram to provide more precise information on where to treat within the vessel. The study will also use iFR to measure if the treatment succeeds in restoring sufficient blood flow to prevent ischemia or if further treatment is warranted.
“iFR continues to be adopted in clinical practice, with mounting evidence that this innovative technology contributes to improved outcomes, reduced costs [2,3,4] and enhanced patient experiences,” said Chris Landon, senior vice president and general manager image guided therapy devices at Philips. “This major study will provide a definitive answer to the question of whether a functional guidance strategy throughout the procedure demonstrates superior outcomes and reduces costs. The study has the potential to drive a significant improvement in clinical practice, and it’s a prime example of Philips’ commitment to providing a strong evidence base for the benefits of its healthcare innovations.”
SyncVision is part of the Philips' portfolio of systems, smart devices, software and services available on its Azurion Image Guided Therapy System. In addition to advanced imaging systems, the portfolio includes coronary imaging catheters, coronary atherectomy and coronary crossing devices, specialty balloons, flow wires, and pressure wires.
The DEFINE GPS study is sponsored by Philips, with the Cardiovascular Research Foundation (CRF) overseeing core lab and clinical event committee activities.
Related iFR, FFR and PCI Guidenace Technology Content:
DEFINE GPS Study Assesses PCI Guided by Integrated iFR and Angiography
FFR Studies Find Several Surprises at TCT 2020
How Interventionalists Can Keep From Missing Coronary Stenoses
Updated SCAI Expert Consensus Says Either FFR, iFR Can Be Used
VIDEO: iFR Equal to FFR Outcomes in Coronary Lesion Evaluation — Interview with Justin Davies, MBBS, and Matthias Götberg, M.D.
New Technology Directions in Fractional Flow Reserve (FFR)
iFR More Cost-Effective Than FFR in PCI Guidance, DAIC
Easier to Use iFR Equal to Outcomes of FFR in Coronary Lesion Evaluation
VIDEO: iFR Found More Cost Effective Over Standard FFR — Interview with Manesh Patel, M.D.
One in Four Patients Have Residual Ischemia Following PCI
References:


 January 05, 2026
January 05, 2026 









