May 7, 2009 - GE Healthcare and Hansen Medical Inc. today said they have entered into a collaboration to promote the use of GE Healthcare’s imaging technologies and Hansen Medical’s robotic catheter technologies for electrophysiology (EP) procedures.
In addition, the companies expect to pursue integrated product offerings in connection with their collaboration. The partnership enables the companies to promote the compatibility of the GE Innova X-ray imaging platform with Hansen Medical’s Sensei Robotic Catheter System. The companies seek to further augment the capabilities of electrophysiologists to perform EP procedures.
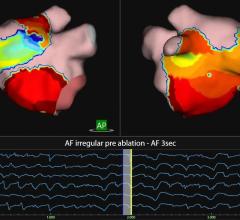
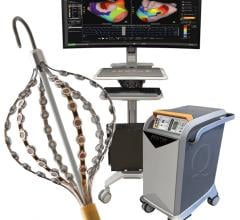
The Sensei system allows physicians to instinctively position robotic catheters in difficult anatomical locations within the heart in a stable and controlled manner, Hansen said. The GE Healthcare Innova imaging system affords clinicians the opportunity to perform angiographic studies efficiently, accurately and reliably during EP and interventional procedures.
The Sensei Robotic Catheter system is a robotic navigation system that enables clinicians to place mapping catheters in hard-to-reach anatomical locations within the heart easily, accurately and with stability during complex cardiac arrhythmia procedures. The Sensei system is compatible with fluoroscopy, ultrasound, 3D surface map and patient electrocardiogram data and was cleared by the FDA in May 2007 for manipulation and control of certain mapping catheters in EP procedures. The safety and effectiveness of the Sensei system for use with cardiac ablation catheters in the treatment of cardiac arrhythmias, including atrial fibrillation (AF), have not been established. The Sensei system is CE marked for use during EP procedures, such as guiding catheters in the treatment of AF.
For more information: www.hansenmedical.com, www.gehealthcare.com


 September 05, 2025
September 05, 2025