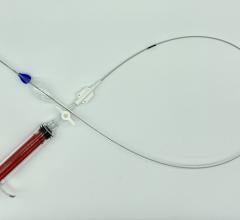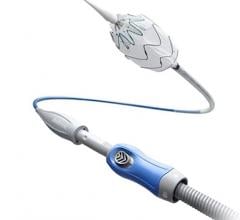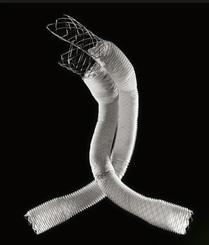
August 19, 2015 — Lombard Medical Inc. a medical device company focused on endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAAs), announced the acquisition of Silicon Valley-based Altura Medical. Altura is a privately-held, venture-backed company that has developed an innovative, ultra-low-profile endovascular stent graft technology that offers a simple and predictable solution for the treatment of standard AAA anatomies.
The terms of the transaction include the issuance of $15 million of Lombard common stock at $4 per share (3,750,000 shares of Lombard common stock subject to certain lock up conditions), the assumption of $5.5 million in bank debt, and $2.5 million in certain liabilities and transaction-related costs.
In addition, up to $27.5 million may be paid based on the achievement of certain commercial and regulatory milestones anticipated over the next five years. Under the terms of the agreement, Lombard has the option to pay the additional consideration in either cash or stock.
The Altura endograft system received CE Mark in 2015 and Lombard plans to launch the device in Europe in January 2016, with a broader international rollout later the same year. In the United States, Lombard intends to file for an IDE (Investigational Device Exemption) from the U.S. Food and Drug Administration (FDA) in early 2016, with the intent to begin recruitment for a U.S. clinical study later in 2016.
“The Altura device offers a new ultra-low-profile stent graft system without compromising the robustness and durability of the wire and graft fabric,” said Prof. Dierk Scheinert, M.D., chairman of the Division of Interventional Angiology, University Hospital Leipzig, Germany. He noted, “The added benefits of this smart system are the ability to reposition during deployment and place each graft accurately to each renal artery, enabling physicians to utilize all the available aortic neck. It also removes the need for cannulation and therefore provides a simple, intuitive, safe and consistent deployment system with predictable and shorter procedure times.”
“Many patients who present for AAA repair can be treated quickly and efficiently with minimal hospital stay and recovery times,” said Stuart A. Harlin, M.D., board certified vascular surgeon, Coastal Vascular & Interventional, Pensacola, Florida. “The introduction of an easy-to-deploy AAA stent graft that offers enhanced safety and accuracy on an ultra-low-profile delivery system will allow physicians to treat a large percentage of AAA patients more efficiently in the future.”
For more information: www.lombardmedical.com, www.alturamed.com


 September 18, 2025
September 18, 2025