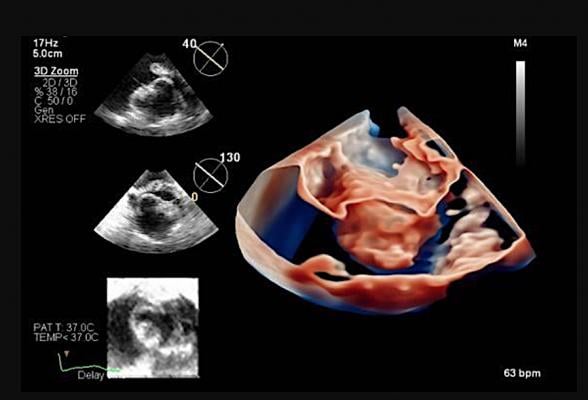
ICE image showing the inside of the heart. Mayo Clinic recently became the first to use the next-generation Philips 4-D intracardiac echo imaging catheter. ICE is seeing increased use in structural heart procedures.
July 16, 2021 — Mayo Clinic recently became the first to use a next-generation 4-D intracardiac echo (ICE) imaging device in a patient to guide a transcatheter left atrial appendage (LAA) closure at Mayo Clinic.
The new Philips Healthcare VeriSight Pro ICE catheter offers superior 2-D and 3-D live image guidance for a wide range of procedures in structural heart disease and electrophysiology, allowing interventionalists to navigate procedures. It can be used to help guide transcatheter mitral valve repair (TMVR) and treatment of tricuspid valve regurgitation. The technology can also help physicians perform procedures without putting patients under general anesthesia.
Live ultrasound imaging during structural heart disease and electrophysiology procedures typically relies on transesophageal echocardiography (TEE) imaging, in which an ultrasound probe is passed down the patient’s throat and into their esophagus, until it lies next to the heart. TEE requires the patient to be sedated or given general anesthesia, lengthening preparation, procedure and recovery times and carrying a degree of risk. Philips’ ICE catheter VeriSight Pro uses the same ultrasound technology, miniaturized to fit on the tip of a 3 mm diameter (9 French) catheter so that it can be navigated to the patient’s heart via their vasculature using the same route used to introduce other catheters during minimally-invasive cardiac interventions. General anesthesia is typically not required, reducing patient risk, and opening up procedures to patients who are not good anesthesia candidates.
"The next generation 4-D ICE technology can transform how we perform some complex transcatheter interventions. For example, it facilitates the performance of left atrial appendage closure under moderate sedation, making the procedure accessible to many patients who are not good candidates for general anesthesia. It also provides excellent imaging of the tricuspid valve, allowing of a more effective transcatheter treatment of tricuspid regurgitation," explains Mohamad Adnan Alkhouli, M.D., an interventional cardiologist at Mayo Clinic in Rochester.
 The new 4-D echo catheter, which is small enough to be inserted into the heart chambers, uses advanced imaging technology to create high-quality 3-D moving images. This technology allows physicians to see structures and blood flow in live motion inside the heart, which is a major improvement over the standard intracardiac echo catheters that are only capable of providing 2-D images.
The new 4-D echo catheter, which is small enough to be inserted into the heart chambers, uses advanced imaging technology to create high-quality 3-D moving images. This technology allows physicians to see structures and blood flow in live motion inside the heart, which is a major improvement over the standard intracardiac echo catheters that are only capable of providing 2-D images.
This in-motion view from inside the heart provides useful information for clinicians, especially in areas of the heart that are difficult to adequately view via transthoracic or transesophageal echo. The 4-D technology provides greater visual details to guide interventional heart procedures, such as left atrial appendage closure, mitral valve repair and treatment of tricuspid valve regurgitation. Also, the 4D echo does not require the patient to be under anesthesia, which is important for patients with sedation sensitivities.
In June 2021, Alkhouli performed the first series of in-person transcatheter interventions at Mayo using 4-D ICE imaging and is working to create standardized imaging protocols for ICE-guided structural heart procedures.
"Mayo Clinic continues to be on the forefront of novel technologies that expand access to care for our growing population of patients with structural heart diseases," Alkhouli said.
For interventional cardiologists, VeriSight Pro delivers a good alternative high quality 3-D live image to TEE, including 3-D Volume and color flow Doppler imaging. Based on Philips’ innovative xMatrix 3D ultrasound transducer technology, Verisight Pro can provide a 90 by 90 degree 3-D field of view as well as allowing physicians to view two scan planes simultaneously. For example, the long and short axes of the LAA to select the appropriately sized device for LAA closure. VeriSight Pro provides superior 2-D ICE imaging, as well as the ability to access new views more easily. Interventionalists can digitally change the scan angle to capture views without manually repositioning the ultrasound tip, a unique feature of this ICE catheter.
The VeriSight Pro is designed to be used with Philips Epiq CVx. The new ICE catheter is available on a limited basis in the U.S.
For more information: www.usa.philips.com/healthcare
Related Intra-cardiac Echo Content:
Northwestern Medicine First in U.S. to Use Live 3D Intracardiac Echo for EP Procedure
Mayo Clinic First to Use Next Generation 4D ICE System to Guide Structural Heart Procedures
Trends and Advancements in Intra-cardiac Echo Imaging Systems
Intra-Cardiac Echo Aids EP Ablations, Structural Heart Procedures
Intracardiac Echo Should be More Widely Adopted to Minimize Radiation in Catheter Ablations


 November 14, 2025
November 14, 2025 









