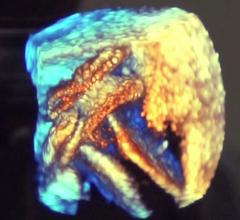
This image showing the aortic and mitral valves in life-like TrueView rendering is from a Philips Epiq CV version 9.0 system.
January 17, 2022 – As the increasing number of structural heart interventions are assisted by real-time imaging guidance, interventional echocardiography is being recognized as a subspecialty requiring advanced training for intra-procedural guidance. A new guideline document from the American Society of Echocardiography (ASE), Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention, focuses on providing a teaching resource for this growing population of specialists.[1]
The guideline elucidates and standardizes the acquisition of essential pre-interventional transesophageal echo (TEE) images to help accurately identify the mechanism of structural/valvular dysfunction, the hemodynamic as well as anatomic severity of the disease, and specific anatomic features to facilitate appropriate device selection or exclusion. This peer-reviewed guideline, created by 14 international echocardiographers who are experts in this emerging field, has been endorsed by 25 ASE International Alliance Partners and is published in the January 2022 issue of the Journal of the American Society of Echocardiography.[1]
"The guideline is intended for the Level II or III echocardiographer whose pre-procedural imaging becomes the backbone of structural heart disease (SHD) procedural planning for the heart team," explained lead guideline author Rebecca T. Hahn, M.D., FASE. "The structured imaging acquisition protocols and extensive figures and tables in this document show examples of the protocols and should benefit all echocardiographers involved in the care of SHD patients. ASE has been an early adopter of the concept that Interventional Echocardiography is a new subspecialty. As with other developing clinical imaging needs, ASE has taken the lead on defining the training and competencies required for interventional echocardiography, and has now taken the first step to standardizing the education around this exciting new field with this guideline."
The guidelines explain how to acquire a comprehensive TEE exam using a standard 28-view imaging protocol as well as specific structural imaging assessments. The ASE said the purpose of the document is to provide a reference guideline focused on the acquisition of essential pre-interventional TEE images that would help identify:
• The mechanism of structural or valvular dysfunction.
• The hemodynamic and anatomic severity of the disease.
• The specific anatomic features that allow appropriate device selection or exclusion.
Intraprocedural imaging, whether by TEE or intracardiac echocardiography, is not covered, but rather a general approach to TEE screening of the structural target. This includes sections on the aortic valve, mitral valve, or tricuspid valve, the left atrial appendage (LAA), and septal defects.
In conjunction with the publication of this guideline as an article in press in November, Hahn conducted two live webinars in December 2021:
Part 1: Introduction to the Guidelines and Review of Aortic and Mitral Imaging
Part 2: Guidelines and Review of Tricuspid and Interatrial Septum/LAA Imaging Protocols
This document and all ASE Guideline documents are also available to the online at ASEcho.org/Guidelines.
For more information: ASEcho.org
Related Interventional Imaging Content:
VIDEO: Transcatheter Structural Heart Procedure Navigation Technology Advances — Interview with Stephen Little, M.D.
The Essentials of Structural Heart Imaging
Requirements for Interventional Echocardiographers
VIDEO: What is Required for Interventional Echo - Interview With Rebecca Hahn, M.D.
VIDEO: Tricuspid Valve Imaging and Interventions Developing Hand-in-hand - Interview With Rebecca Hahn, M.D.
VIDEO: Role of Interventional Echocardiography in Transcatheter Structural Heart Procedures - Interview With Rebecca Hahn, M.D.
Reference:


 June 20, 2024
June 20, 2024