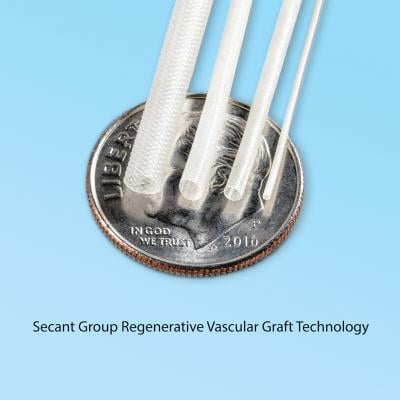
January 17, 2018 — Secant Group, in partnership with its sister company SanaVita Medical, announced new technology to advance cardiovascular regenerative medicine with the development of a synthetic, small-bore vessel that encourages endogenous regeneration and new vessel formation. The technology is based on the company’s textile forming capabilities that can produce a hollow lumen construct infused with Secant’s proprietary Regenerez bioresorbable polymer technology. The new small-bore vessel supports the regeneration of new vascular tissue structures without the need for cell seeding or biologic growth promoters.
In situ vascular regeneration, along with the elastomeric and immunomodulatory properties of the synthetic vessel, could solve the problems of vascular harvesting and the non-resorbable synthetic graft compliance mismatching seen with the current technology available for surgeons today. These regenerative grafts will offer new benefits for coronary artery bypass surgery, peripheral vascular disease and renal disease treatments.
Secant has produced small-bore grafts with lumen diameters down to 500 μm, closely matching the range of human vessels. Jeff Robertson, president of Secant Group, explained that by combining Secant’s textile manufacturing and biomaterials capability, this small-bore graft would provide similar compliance as native vasculature, enabling a seamless connection between graft and vessel.
Devices that currently dominate the cardiovascular graft replacement market have numerous limitations including compliance mismatch, low patency rates, calcification, a risk of infection and lack of tissue regeneration capability. The Secant small-bore graft addresses many of these limitations. Early pre-clinical studies have confirmed that Regenerez grafts are non-thrombogenic, did not show occlusion and provide excellent suturability. Secant is continuing the development of small-bore grafts in collaboration with a leading heart and vascular research university. The company is currently looking for medical device partners to progress the technology through clinical trials and commercialization.
For more information: www.secant.com


 January 15, 2026
January 15, 2026 









