April 11, 2008 - St. Jude Medical will acquire EP MedSystems for approximately $92.1 million in a deal that will accelerate St. Jude Medical's atrial fibrillation program and entry into the high-growth intracardiac ultrasound echocardiography (ICE) market.
Under the terms of the merger agreement, EP MedSystems shareholders will receive $3.00 of consideration for each EP MedSystems share they own, with the option of receiving that amount in cash or St. Jude Medical common stock.
In connection with this transaction, St. Jude Medical's Board of Directors has approved an additional stock buyback authorization of $50 million, which increases St. Jude Medical's share repurchase authorization from $250 million to $300 million. The additional buyback authorization will be used to offset the shares issued in this transaction.
The companies anticipate this acquisition will close during the third quarter of 2008. In connection with this transaction, St. Jude Medical will record a special charge for in-process research and development. This acquisition does not change St. Jude Medical's existing guidance for 2008 earnings per share, exclusive of the special charge.
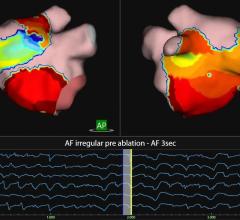
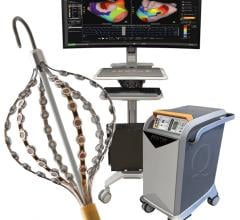
Upon completion, this transaction will immediately add two new growth drivers to St. Jude Medical's program for products used in atrial fibrillation (AF) and other electrophysiology (EP) catheterization procedures. This includes the EP-WorkMate computerized electrophysiology workstation with a fully integrated EP-4 Computerized Cardiac Stimulator and expansion options to incorporate the NurseMate Remote Review Charting Station. The EP-WorkMate platform already enjoys a strong number two share of the global market for EP recording systems in spite of limited sales and marketing resources.
This transaction will also expedite St. Jude Medical's entry into the high-growth intracardiac ultrasound echocardiography (ICE) market with the EP MedSystems ViewMate II intracardiac ultrasound system and the next generation ViewFlex PLUS ICE catheter scheduled for market release this quarter. This market is growing at an estimated 25 percent to 30 percent per year and includes both electrophysiology and interventional cardiology applications.
The transaction is subject to certain closing conditions and regulatory approvals, and approval by EP MedSystems shareholders. Following the close of the transaction, Bruce is expected to join St. Jude Medical, and EP MedSystems will become part of the Atrial Fibrillation division of St. Jude Medical.
For more information: www. sjm.com


 September 05, 2025
September 05, 2025