December 23, 2008 - St. Jude Medical Inc. and MediGuide Inc. today signed a definitive merger agreement where MediGuide will become part of the St. Jude Medical Atrial Fibrillation Division.
St. Jude Medical has acquired all of the outstanding shares of MediGuide Inc., including the 41.3 percent interest for $283 million in cash and the assumption of net liabilities totaling approximately $17 million. Terms of the transaction provide for St. Jude Medical to pay $138 million of the purchase price in December 2008, with the balance due in two subsequent payments of $111 million in November 2009 and up to $34 million in April 2010.
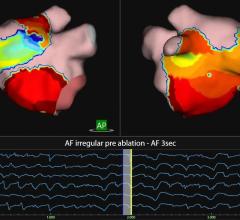
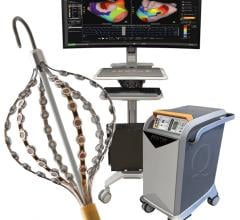
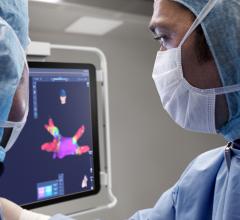
MediGuide has developed a sophisticated navigation system, the Medical Positioning System (gMPS), that uses proprietary technology for real-time tracking of sub-millimeter sized sensors. These sensors can be mounted on needles, guide wires, catheters, or other medical devices used for minimally-invasive intra-body navigation. The 3D position and orientation of the sensors can be calculated in real time and projected graphically on a fluoroscope, CT, MRI, ultrasound, or 3-D reconstructed image of the anatomy. The gMPS system is intended to provide a comprehensive solution for motion artifacts caused by patient heartbeats, respiration, or other movements. The goal of the gMPS system is to increase the accuracy and amount of information available to a physician during a catheterization or other minimally invasive procedure while reducing the risk of exposure to radiation for the patient and medical team. MediGuide's gMPS technology and its gMPS Enabled Guided Measurement Catheter (GMC) are European CE Mark certified and are currently limited to investigational use only in the U.S.
For more information: www.sjm.com


 September 05, 2025
September 05, 2025