October 2, 2017 — Cardiovascular healthcare membership organization and performance community MedAxiom announced its ...
October 2, 2017 — Reflow Medical Inc. announced that the company has received 510(k) clearance from the U.S. Food and ...
Medtronic plc recently announced a new post-market clinical study to evaluate its CoreValve Evolut Pro valve in everyday clinical practice. Studying patients with severe symptomatic aortic stenosis at an intermediate, high or extreme risk for open-heart surgery, the FORWARD PRO Clinical Study will evaluate longer-term performance (out to five years) of the next-generation self-expanding transcatheter aortic valve implantation (TAVI) system, which was recently approved for commercial use in Europe and United States.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 2, 2017 — Boston Scientific announced a definitive agreement to acquire Apama Medical Inc., a privately-held ...

October 2, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
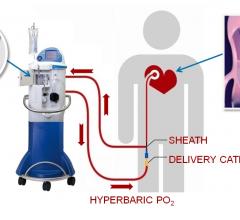
September 29, 2017 — TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the premarket ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Avinger Inc. recently announced Conformité Européenne (CE) Marking approval for treating in-stent restenosis with the Pantheris Lumivascular atherectomy system.
September 28, 2017 — New research revealed that on average, more than 75 percent of people aged 65 and older worldwide ...
Farouc Jaffer, M.D., Ph.D., director of coronary interventions at Massachusetts General Hospital, discusses the newest ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
September 28, 2017 — The AstraZeneca HealthCare Foundation’s Connections for Cardiovascular Health (CCH) program is ...
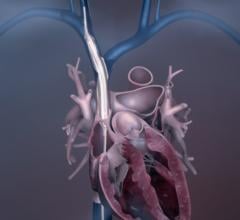
Abiomed Inc. recently received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for the Impella RP heart pump. Culminating from five years of research, this approval follows the prior FDA Humanitarian Device Exemption (HDE) received in January 2015 and adds the Impella RP heart pump to Abiomed's platform of PMA-approved devices.
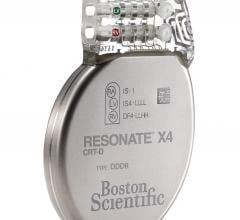
September 27, 2017 — Boston Scientific recently launched the Resonate family of implantable cardioverter defibrillator ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Toshiba Medical announced it will highlight several of its latest vascular and interventional imaging solutions at the 2017 Annual Meeting of the Radiological Society of North America (RSNA), Nov. 26-Dec. 1 in Chicago.
Spectranetics is recalling its Bridge Occlusion Balloon Catheter due to the possibility of a blocked guidewire lumen in some device units. If a device with a blocked guidewire lumen were to be used during the procedure, the device would not be positioned correctly and hemorrhage would not be controlled. This would delay life-saving treatment, which may result in immediate and serious adverse health consequences, including death.
BioCardia Inc. recently announced 12-month results from the Phase II TRIDENT clinical trial, conducted by the University of Miami Miller School of Medicine and co-sponsored by the company. The results showed a positive safety profile for allogeneic, or donor cell-based, mesenchymal stem cells delivered with the company’s Helix transendocardial delivery system at 30 days. The study was concurrently published in Circulation Research and presented on the podium at the Heart Failure Society of America (HFSA) Annual Scientific Meeting, Sept. 16-19 in Dallas. Results were presented by Victoria Florea, M.D., of the Interdisciplinary Stem Cell Institute at the University of Miami.

 October 02, 2017
October 02, 2017