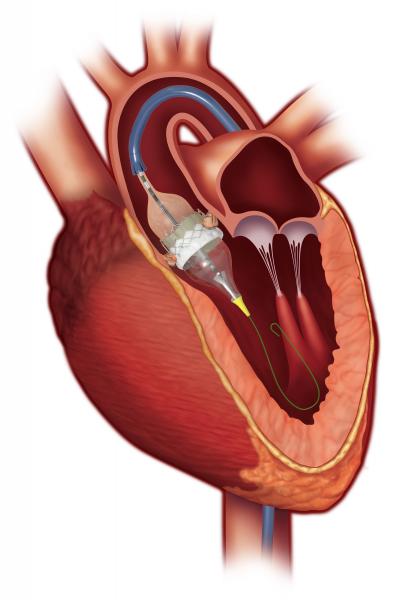
The U.S. Food and Drug Administration (FDA) has granted market clearance for aortic and mitral valve-in-valve procedures using the Edwards Lifesciences Sapien 3 transcatheter heart valve (THV). The Sapien 3 valve is the first transcatheter heart valve approved in the U.S. for the treatment of both aortic and mitral patients who are at high risk for a subsequent open-heart surgery to replace their bioprosthetic valve.
June 1, 2017 — More than 50 companies and organizations will display their latest products and services at the American ...
June 1, 2017 — Here is the list of the most popular pieces of content on the Diagnostic and Interventional Cardiology ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Northwell Health recently announced a new partnership agreement with Peerbridge Health Inc., to explore the future of remote medical monitoring. Peerbridge technology enables remote patient monitoring through multiple on-body wireless sensors.
June 1, 2017 — Infab Corp. recently introduced Revolution Lead and Lead-Free Aprons with Kiarmor, Infab’s proprietary bi ...
Lucas Boersma, M.D., Ph.D., FESC, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands, discusses how subcutaneous ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

ASE Announces Meeting Highlights for 28th Annual Scientific Sessions — The program includes learning opportunities from ...
LivaNova PLC recently announced the presentation of data from multiple studies demonstrating the safety and effectiveness of the PercevalM sutureless valve for aortic valve replacement (AVR) patients and the Memo 3D ReChord for mitral valve repair. The three data presentations on Perceval, which included a late-breaking clinical trial and a poster presentation on the Memo 3D ReChord, were unveiled at the American Association for Thoracic Surgery (AATS) Centennial meeting in Boston, April 29 – May 3.
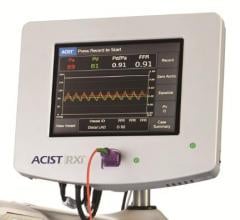
Acist Medical Systems Inc. announced results from the ACIST-FFR Study demonstrating the consistent and correlative performance of the Navvus MicroCatheter compared to standard pressure wire systems. ACIST-FFR (Assessment of Catheter-based Interrogation and Standard Techniques for Fractional Flow Reserve measurement) is the largest multi-center study to compare microcatheter FFR to pressure wire FFR, as well as the only study to include multiple pressure wire comparators. The study was presented for the first time at EuroPCR 2017, May 16-19 in Paris, France.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
MIM Software Inc. recently announced significant updates to its MIM Encore solution for viewing nuclear medicine images. The vendor-neutral application offers viewing of positron emission tomography (PET)/computed tomography (CT), single photon emission computed tomography (SPECT)/CT, PET/magnetic resonance imaging (MRI) and general nuclear medicine images. Physicians and technologists can streamline the nuclear medicine workflow by viewing and processing images in tandem using this single-platform solution.
BioVentrix Inc. recently announced the first clinical use of its closed-chest Revivent TC TransCatheter Ventricular Enhancement System at the Deutsches Herzzentrum Berlin in Germany. The Less Invasive Ventricular Enhancement (LIVE) procedure was performed by interventional cardiologist Christoph Klein, M.D., and cardiothoracic surgeon Felix Hennig, M.D., with important contributions from Sebastian Kelle, M.D., chief of cardiovascular imaging.
May 31, 2017 – The results from the first real-world, matched head-to-head study comparing all-cause healthcare costs ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
A Canadian cardiologist has published a report in the journal Eurointervention describing how he used a Canadian-invented device for the first time in the world to successfully insert a MitraClip through a patient's jugular vein rather than the femoral vein.
The U.S. Food and Drug Administration (FDA) announced that it has granted market clearance to Ra Medical Systems for the company’s DABRA (Destruction of Ateriosclerotic Blockages by laser Radiation Ablation) System.
Early results from the independent, physician-sponsored FFR-Search Registry revealed an association between post-percutaneous coronary intervention (PCI) fractional flow reserve (FFR) measurements with the Acist Navvus Rapid Exchange FFR MicroCatheter and clinical outcomes. The results reveal a potential new role for FFR in cath labs.

 June 06, 2017
June 06, 2017