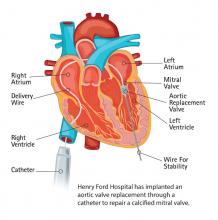
Vascular Solutions Inc. launched the ThrombiDisc topical hemostat designed for use around indwelling lines up to 12°F. ThrombiDisc contains both the power of thrombin to facilitate hemostasis and the antimicrobial properties of silver.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now