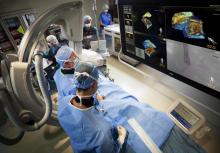
Toshiba America Medical Systems Inc. offers electrophysiology (EP) clinicians an Infinix-i cardiovascular X-ray system tailored for EP procedures with a new package of features, accessories and technologies. This package maximizes room utilization, improves workflow and enhances safety.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now