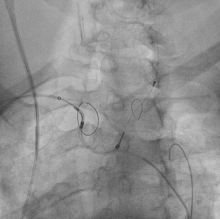
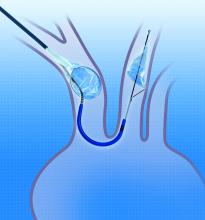
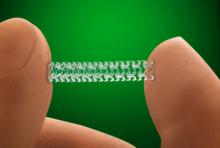
October 14, 2015 — Claret Medical, an innovator in cardiovascular cerebral protection, Data presented this week at Transcatheter Cardiovascular Therapeutics (TCT) 2015 showed the first definitive cognitive benefit from use of a cardiovascular cerebral protection system during transcatheter aortic valve replacement (TAVR) procedures.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now