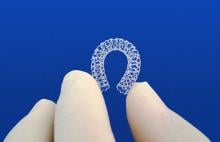
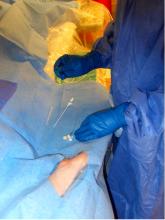
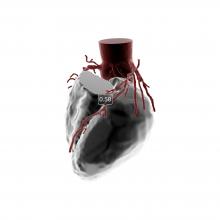
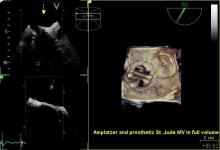
Results from the BRAVO 3 trial found that bivalirudin did not significantly reduce major bleeding rates at 48 hours or adverse events at 30 days compared to heparin in high-risk patients undergoing transcatheter aortic valve replacement (TAVR).
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now