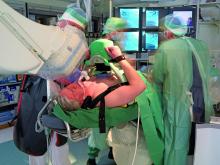
Abiomed Inc. said the Impella RP (Right Percutaneous) system has received U.S. Food and Drug Administration (FDA) approval under a humanitarian device exemption (HDE). This is the first percutaneous single access heart pump designed for right heart support to receive FDA approval. Abiomed completed the HDE submission for the Impella RP in September 2014 following the completion of the RECOVER RIGHT study.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now