May 14, 2012 — During the Heart Rhythm Society (HRS) 2012 scientific sessions last week, Biosense Webster Inc. announced it created strategic collaboration agreements with GE Healthcare and Siemens Healthcare. The agreements will make it easier for the vendors to work jointly to create advanced electrophysiology (EP) labs
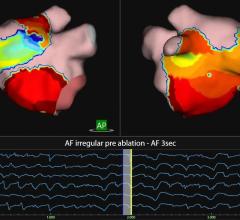
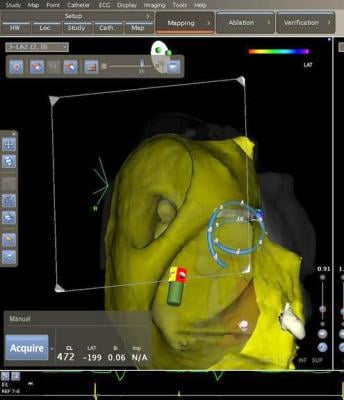
Biosense Webster, Inc. has developed an image integration solution for electrophysiology (EP) labs around the world. This solution integrates real-time X-ray images, from Siemens Healthcare and GE Healthcare angiography systems, into the company’s Carto 3 3-D electro-anatomical mapping and ablation system. Under the terms of the two agreements, Biosense Webster plans to offer an integrated solution, compatible with both manufacturers’ fluoroscopy systems in 2012.
“Minimizing X-ray exposure for patients and clinicians has long been a focus of healthcare technology manufacturers,” said Shlomi Nachman, worldwide president, cardiovascular care franchise. “These two important strategic agreements will allow Biosense Webster to address the growing need to combine multiple images from different systems in the EP lab into a single display on our CARTO 3 system.”
These latest agreements mark a significant step towards providing a comprehensive, integrated lab solution to electrophysiologists and hospitals around the world. GE Healthcare and Siemens Healthcare collectively, represent more than 50 percent of the installed base of X-ray systems across the globe.
“These strategic collaborations and the resulting technology advancements, continue our commitment to bringing innovation to the cardiac electrophysiology community so they may better serve their patients and protect themselves,” said Nachman.
For more information: www.biosensewebster.com


 September 05, 2025
September 05, 2025