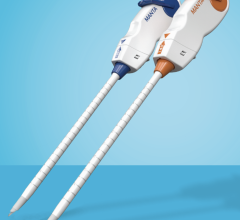
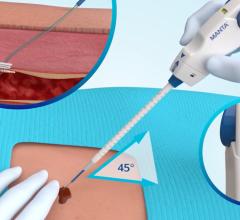
The StarClose SE Vascular Closure System is a next-generation vessel closure device engineered to enable fast and secure closure of the femoral artery access site following a catheterization procedure.
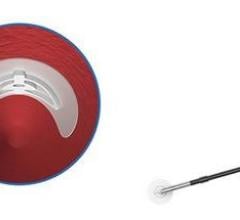
Available in the U.S. and Europe, StarClose SE (safe and extravascular) builds upon the design of its predecessor, StarClose, reportedly with more ergonomically friendly design features. StarClose SE utilizes the same nitinol (nickel and titanium) clip technology as StarClose to close the femoral artery access site after a catheterization procedure. When deployed, the small nitinol clip grasps the tissue on top of the artery around the access site in a purse-string fashion, and closes the opening in the femoral artery rapidly and securely with minimal affect to the lumen diameter or the blood flow inside the vessel.
StarClose SE advancements include an intuitive, numbered system, providing visual guidance and audible clicks for each step leading to clip deployment; immediate vessel closure with deployment of a shape-memory clip onto the surface of an artery; and added device stability for the operator during clip deployment.
The StarClose was launched in the United States in May 2008.
For more information: www.abbott.com
Related Vascular Closure Content
Devices to Aid Hemostasis Speed Ambulation, Reduce Staff Time
Justifying the Use of Vascular Closure Devices
Vascular Closure Devices Show Safety Comparable to Manual Compression

 October 07, 2025
October 07, 2025