September 10, 2020 - Icosapent ethyl (Vascepa) demonstrated significant, 17 percent regression of low attenuation plaque ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
September 8, 2020 - Heart patients hospitalized with COVID-19 (SARS-CoV-2) can safely continue taking angiotensin ...
September 3, 2020 — Why so many COVID-19 (SARS-CoV-2) patients get blood clots (thrombosis) remains uncertain. But ...
September 2, 2020 — An international, first-of-its-kind cardiology trial used personalized genetic testing to reduce by ...
July 28, 2020 — AstraZeneca's heart failure medication dapagliflozin (Farxiga) significantly reduced the worsening of ...
July 24, 2020 — CeloNova BioSciences Inc. announced it successfully completed enrollment of the COBRA REDUCE randomized ...

June 15, 2020 — The U.S. Food and Drug Administration (FDA) today revoked the emergency use authorization (EUA) that ...
Interview with Andrew D. Krahn, M.D.,FHRS, head of the division of cardiology at St. Paul’s Hospital, and professor of ...
May 27, 2020 — Two of the front-line drugs being used to treat COVID-19 (SARS-CoV-2) patients are hydroxychloroquine and ...
Marianne Pop, Pharm.D., BCPS, a clinical pharmacist and clinical assistant professor with the regional pharmacy program ...
May 17, 2020 – Patients with high lipid levels have an increased risk for ischemic events, despite statin therapy ...
May 17, 2020 – A new study sought to reveal whether drug-eluting stents (DES) coated with bioabsorbable polymer (BP) ...
May 13, 2020 — The patient-administered nasal spray drug etripamil did not meet its primary endpoint in treating ...
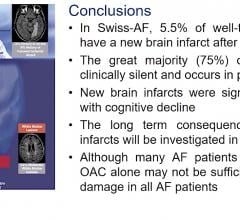
May 8, 2020 – A new clinical study found that patients with atrial fibrillation (AF) experienced a high incidence of ...

April 23, 2020 — The U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication today reminding doctors ...


 September 10, 2020
September 10, 2020