There have been several safety reports to the FDA that COVID-19 patients receiving hydroxychloroquine and chloroquine, either alone or combined with azithromycin or other QT prolonging medicines, have caused serious These adverse events, including death. Events reported included QT interval prolongation, ventricular tachycardia and ventricular fibrillation.
April 23, 2020 — The U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication today reminding doctors there are serious side effects when using hydroxychloroquine and chloroquine in the off-label use to treat COVID-19 patients. This includes potentially life-threatening heart rhythm problems.
The FDA said case reports from the FDA Adverse Event Reporting System database, published medical literature and the American Association of Poison Control Centers National Poison Data System are reporting serious heart-related adverse events and death in patients.
These COVID-19 patients were receiving hydroxychloroquine and chloroquine, either alone or combined with azithromycin or other QT-prolonging medicines. These adverse events included QT interval prolongation, ventricular tachycardia and ventricular fibrillation, and in some cases, death, the FDA said in its safety report. The FDA also said patients who have other health issues, such as heart and kidney disease, are at increased risk of these heart problems when receiving these medicines.
“We understand that healthcare professionals are looking for every possible treatment option for their patients and we want to ensure we’re providing them with the appropriate information needed for them to make the best medical decisions,” said FDA Commissioner Stephen M. Hahn, M.D. “While clinical trials are ongoing to determine the safety and effectiveness of these drugs for COVID-19, there are known side effects of these medications that should be considered. We encourage healthcare professionals making individual patient decisions closely screen and monitor those patients to help mitigate these risks. The FDA will continue to monitor and investigate these potential risks and will communicate publicly when more information is available.”
The FDA says it has received several reports of safety issues involving patients due to the use of hydroxychloroquine and chloroquine for the treatment or prevention of novel coronavirus (COVID-19, SARS-CoV-2), for which they are not approved by the FDA.
The agency said the risks, which are in the drug labels for their approved uses, may be mitigated when healthcare professionals closely screen and supervise these patients such as in a hospital setting or a clinical trial, as indicated in the Emergency Use Authorization (EUA) for these drugs to treat COVID-19.
The FDA has issued an EUA to allow hydroxychloroquine and chloroquine products donated to the Strategic National Stockpile (SNS) to be distributed and used in limited circumstances, such as for certain hospitalized patients with COVID-19. These drugs are able to be distributed from the SNS to states for doctors to prescribe to adolescent and adult patients hospitalized with COVID-19, as appropriate, when a clinical trial is not available or feasible. The EUA requires that fact sheets with important information about using these drugs in treating COVID-19, including the known risks and drug interactions, as well as appropriate screening and monitoring, be made available to healthcare providers and patients.
FDA Adverse Event Reports Echo Warnings by Cardiology Societies
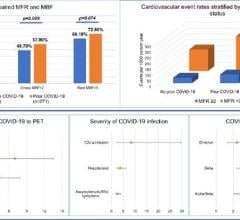
The negative cardiovascular impact of these drugs were included in an official warning in a joint statement April 8 from the American Heart Association (AHA), the American College of Cardiology (ACC) and the Heart Rhythm Society (HRS). These societies jointly published the guidance document, “Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 19) Treatment,” to detail critical cardiovascular considerations in the use of hydroxychloroquine and azithromycin for the treatment of COVID-19.[1]
The antimalarial medication hydroxychloroquine and the antibiotic azithromycin are both being used as potential treatments for COVID-19, and each have potential serious implications for people with existing cardiovascular disease. The societies warned complications include severe electrical irregularities in the heart such as arrythmia (irregular heartbeat), polymorphic ventricular tachycardia (including Torsade de Pointes) and long QT syndrome, and increased risk of sudden death. The effect on QT or arrhythmia of these two medications combined has not been studied.
The AHA, the ACC and the HRS guidance for health care professionals includes additional mechanisms to reduce the risk of arrhythmias. Steps outlined:
• Electrocardiographic/QT interval monitoring;
• Withhold hydroxychloroquine and azithromycin in patients with baseline QT prolongation (e.g. QTc ≥500 msec) or with known congenital long QT syndrome;
• Monitor cardiac rhythm and QT interval; withdrawal of hydroxychloroquine and azithromycin if QTc exceeds a present threshold of 500 msec;
• In patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible;
• Correction of hypokalemia >4mEq/L and hypomagnesemia >2mg/dL; and
• Avoid other QTc prolonging agents whenever feasible.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” said Robert A. Harrington, M.D., FAHA, president of the American Heart Association, Arthur L. Bloomfield Professor of Medicine and chair of the department of medicine at Stanford University.
Read more in the AHA, ACC, HRS Caution Use of COVID-19 Therapies Hydroxychloroquine and Azithromycin in Cardiac Patients
FDA Approved Indications and COVID-19 Trials for Hydroxychloroquine and Chloroquine
Hydroxychloroquine and chloroquine are FDA-approved to treat or prevent malaria. Hydroxychloroquine sulfate is also FDA-approved to treat lupus and rheumatoid arthritis.
The FDA said these medicines have not been proven safe or effective for treating COVID-19. However, clinical trials are underway and additional trials are being planned to determine if these drugs can benefit patients with COVID-19. These trials are also examining whether the drugs can prevent COVID-19 among healthcare workers, first responders or people who have been in close contact with someone with COVID-19.
One of these trials is being run by Henry Ford Hospital in partnership with four other healthcare systems in the Detroit metro area. The 3,000 patient study started in mid-April to test if hydroxychloroquine can help prevent the onset of COVID-19 in front-line clinicians, first responders and bus drivers who do not yet have the virus.
"There is this brewing controversy about whether hydroxychloroquine should be used because it has an arrhythmogenic effect," said cardiologist William O’Neill, M.D., who is heading the trial. "There are two camps. There are the QT experts who live and die QT, and they say 'oh my god, these drugs will cause QT prolongation.' And then there are other experts, including the rheumatology association and the Lupus Foundation that say 'look, we have been using this as an outpatient drug fro decades and we have never seen any kind of cardiac problems.' And that is where we have been meeting some headwinds because some people have concern who say you can use this drug without active EKG monitoring."
He noted millions of healthy patients have used these drugs to prevent malaria effectively and there have not been massive numbers of people reporting arrhythmias.
He said the Detroit trial is screening patients to make sure they do not have any cardiac issues and that they have regular cardiac rhythm prior to entering the trial.
"There are certain segments of the EP community that are horrified we are this drug in outpatients without QT interval monitoring, so I have stepped into this big controversy" O'Neill said.
The FDA warning brough an immediate reaction from would-be trial participants, with the trial going from a couple hundred people interested a day to less than 20 a day. Henry Ford has concerns it might not be able to recruit the 3,000 patients needed to power the trial.
Read the article First Large-scale U.S. Study on Hydroxychloroquine COVID-19 Prophylaxis Begins in Detroit.
Watch the VIDEO: Cardiologists Manage Trial Testing if Hydroxychloroquine Protects Clinicians From COVID-19, interview with William O'Neill, M.D.
Once the FDA has approved a drug, healthcare providers generally may prescribe or administer the drug for an unapproved use, including in clinical settings not described in the approved labeling. This decision will be based on their assessment of the potential benefits versus the risks for their patient, recognizing that the FDA has not assessed the safety or effectiveness of such use. For this reason, it is important that health care providers are aware of the risks of serious and potentially life-threatening heart rhythm problems that can occur with these drugs and are included in the drug labels for their approved uses.
FDA Warns Not to Use Hydroxychloroquine or Chloroquine for COVID-19 Outside Hospital Setting
The FDA cautions against use hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems.
The agency said it is concerned these drugs are being used inappropriately, outside of a clinical setting or trial, to treat non-hospitalized patients for COVID-19 or to prevent that disease. The agency said it authorized their temporary use only in hospitalized patients with COVID-19 when clinical trials are not available, or participation is not feasible, through an EUA.
FDA Warns Consumers About Online Medications and Not to Take Hydroxychloroquine Without Physician Supervision
The FDA issued the following recommendations to consumers about these drugs:
• Do not buy these medicines from online pharmacies without a prescription from your healthcare professional.
• Do not take any form of hydroxychloroquine or chloroquine that has not been prescribed for you by a healthcare provider. Serious poisoning and death have been reported after mistaken use of a chloroquine product not intended to be taken by humans.
If you have these medicines in your home, keep them in childproof containers out of the reach of children to prevent accidental poisoning.
FDA Precautions for Health Professionals Monitoring Hydroxychloroquine Patients
The FDA recommends initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials to treat or prevent COVID-19. Monitoring may include baseline ECG, electrolytes, renal function and hepatic tests.
If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, the FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and considering enrolling the patient. Clinicians should also consider using resources available to assess a patient’s risk of QT prolongation and mortality.
Risks From Taking Hydroxychloroquine and Chloroquine
The FDA reminded physicians in its statement to be aware that hydroxychloroquine or chloroquine can cause the following known adverse effects:
• Cause QT prolongation during the cardiac cycle on ECGs.
• Increase the risk of QT prolongation in patients with renal insufficiency or renal failure.
• Increase insulin levels and insulin action causing increased risk of severe hypoglycemia.
• Cause hemolysis in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
• Interact with other medicines that cause QT prolongation even after discontinuing the medicines due to their long half-lives of approximately 30-60 days.
FDA Warns Patients to Call 911 If They Have Adverse Effects
In its MedWatch statement, it reminds the public to be aware that there are no proven treatments for COVID-19 and no vaccine. If a person is receiving hydroxychloroquine or chloroquine for COVID-19 and experience irregular heartbeats, dizziness or fainting, the FDA said they should immediately seek medical attention by calling 911.
FDA Wants Reports of Adverse Reactions from COVID-19 Treatments
To better understand the the impact of prescribing these and other drugs for COVID-19, the FDA encourages healthcare professionals and patients to report adverse reactions or quality problems with any human drugs to the agency’s MedWatch Adverse Event Reporting program at www.fda.gov/medwatch/report.htm. People cal also call 1-800-332-1088.
Other Impact of COVID-19 on Cardiology Content:
UPDATED JUNE 15, 2020 — FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine for COVID-19
VIDEO: Why QT-prolongation Occurs in COVID-19 Patients on Hydroxychloroquine — Interview with Andrew Krahn, M.D.
COVID-19 Hydroxychloroquine Treatment Brings Prolonged QT Arrhythmia Issues
VIDEO: Overview of Hydroxychloroquine and FDA Warning in its use to Treat COVID-19 — Interveiew with Marianne Pop, Pharm.D.
FDA Grants Emergency Use Authorization to VitalConnect for Cardiac Monitoring in COVID-19 Patients
FDA Reports of Deaths and Injuries From Use of Antimalarial hydroxychloroquine in COVID-19 Patients
VIDEO: Cardiologists Manage Trial Testing if Hydroxychloroquine Protects Clinicians From COVID-19
First Large-scale U.S. Study on Hydroxychloroquine COVID-19 Prophylaxis Begins in Detroit
ESC Council on Hypertension Says ACE-I and ARBs Do Not Increase COVID-19 Mortality.
No Evidence Supporting Discontinuing RAAS Inhibitors in COVID-19 Patients in NEJM Article
ESC Council on Hypertension Says ACE-I and ARBs Do Not Increase COVID-19 Mortality
How to Manage AMI Patients During the COVID-19 Pandemic
VIDEO: Impact of COVID-19 on the Interventional Cardiology Program at Henry Ford Hospital — Interview with William O'Neill, M.D.
VIDEO: 9 Cardiologists Share COVID-19 Takeaways From Across the U.S.
VIDEO: Multiple Cardiovascular Presentations of COVID-19 in New York — Interview with Justin Fried, M.D.
Image Gallery Showing Impact of the COVID-19 Pandemic
ACC COVID-19 Clinical Guidance For the Cardiovascular Care Team
VIDEO: COVID-19 Precautions for Cardiac Imaging — Interview with Stephen Bloom, M.D.
FDA Approves ECMO to Treat COVID-19 Patients
COVID-19 STEMI Registry Created to Study Acute Cardiovascular Effects of the Virus
VIDEO: Best Practices for Nuclear Cardiology During the COVID-19 Pandemic
VIDEO: What Cardiologists Need to Know about COVID-19 — Interview with Thomas Maddox, M.D.
The Cardiac Implications of Novel Coronavirus
Reference:

 March 20, 2024
March 20, 2024