The U.S. Food and Drug Administration (FDA) cleared Carestream Health’s Vue Motion …
HealthMed Holdings completed validation on a …
The U.S. Food and Drug Administration (FDA) granted AliveCor Inc. over-the-counter (OTC)…
Wolters Kluwer Health, a global provider of information for healthcare professionals and…
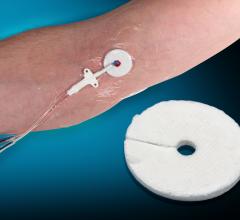
Vascular Solutions Inc. launched the ThrombiDisc topical …
To address the needs of physicians who treat patients with valvular heart disease, Esaote North…
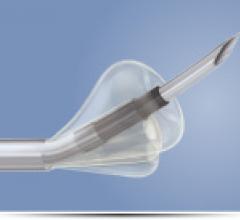
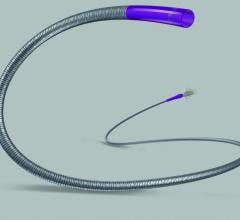
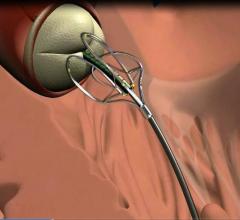
Boston Scientific launched in the United States the OffRoad Re-Entry …
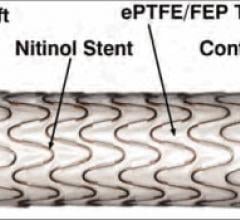
January 28, 2014 — The U.S. Food and Drug Administration (FDA) approved W. L. Gore &…
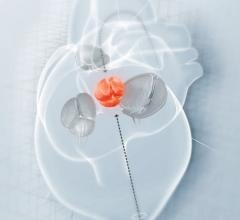
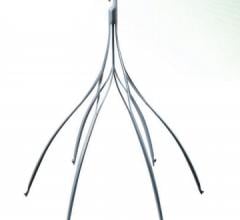
AtheroMed, a developer of …
Boston Scientific has received U.S. Food and Drug Administration (FDA) clearance and CE mark…
The U.S. Food and Drug Administration (FDA) cleared Radius Medical LLC’s Prodigy Support…
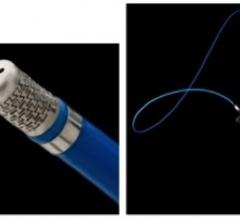
Argon Medical Devices Inc. received clearance from the U.S. Food and Drug Administration (FDA)…
The U.S. Food and Drug Administration (FDA) gave market clearance to Irvine Biomedical Inc., a…
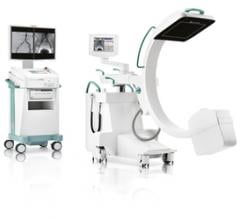
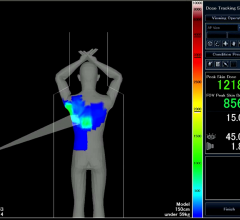
Toshiba America Medical Systems’ Dose Tracking System allows clinicians to track …
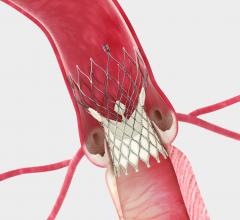
The U.S. Food and Drug Administration (FDA) granted U.S. market approval for Medtronic’s self-…
MediValve Ltd. announced that it has received clearance of a Pre-Marketing Notification…
Biodex Medical Systems Inc. introduced its line of …


 February 18, 2014
February 18, 2014