March 26, 2019 — Level Ex, creators of medical video games for physicians, expanded into…
The U.S. Food and Drug Administration (FDA) approved Impulse Dynamics’ Optimizer Smart system…
March 15, 2019 – Biotronik announced U.S.
A new dosimetry monitoring service from Thermo Fisher Scientific enables medical and imaging…
March 5, 2019 – The U.S.
March 5, 2019 — Connected healthcare solutions provider VivaLNK announced its internet of things…
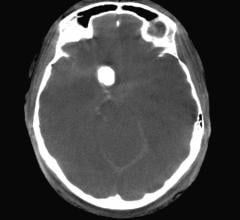
iSchemaView announced the release of RAPID Angio, a complete neuroimaging solution for the…
Philips announced the launch of IntelliSpace Portal 11, the latest release of the company’s…
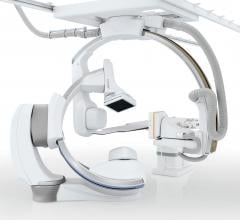
Philips announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile…
Medical imaging and visualization company Medivis officially unveiled SurgicalAR, its augmented…
Philips announced the launch of IntelliSpace Cardiovascular 4.1, its next-generation…
Information technology (IT) services provider Atos announced the launch of its first…
Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for the Valiant…
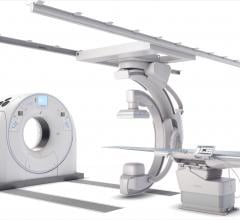
Canon Medical Systems USA Inc. recently introduced a new angiography configuration featuring its…
Canon Medical Systems USA recently introduced its next generation of interventional systems –…
Paragonix Technologies Inc. recently received clearance from the U.S. Food and Drug…
Merit Medical Systems Inc. announced that the PreludeSync Distal Compression Device is now…
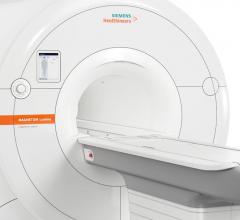
The U.S. Food and Drug Administration (FDA) has cleared the Magnetom Lumina 3 Tesla (3T)…
XableCath Inc., announced that its XableCath blunt and abrasion tip catheters were cleared by…
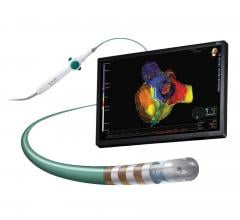
Abbott announced U.S. Food and Drug Administration (FDA) approval of the TactiCath Contact Force…


 March 26, 2019
March 26, 2019