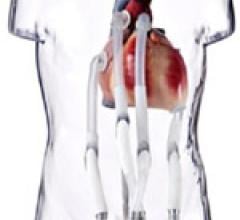
SynCardia Systems Inc. received approval in July from the U.S. Federal Drug Administration (FDA) for the SynCardia temporary Total Artificial Heart with SynHall valves, giving the company control over the last key component to manufacture the Total Artificial Heart
Running for only a few minutes a day or at slow speeds may significantly reduce a person’s risk of death from cardiovascular disease compared to someone who does not run, according to a clinical study published in the Journal of the American College of Cardiology.
Biotronik announced the completion of patient enrollment in the superficial femoral artery (SFA) arm of its BIOFLEX-I clinical trial, an U.S. Food and Drug Administration (FDA)-approved investigative device exemption (IDE) trial evaluating the use of self-expanding nitinol stents in treating peripheral artery disease.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 7, 2014 — The Berlin Heart Group announced they have completed enrollment in their post-approval study, the only condition of the humanitarian device exemption (HDE) approval that Berlin Heart received for the Excor pediatric ventricular assist device (VAD) on Dec. 16, 2011.

August 7, 2014 — A newly released study by IMV Medical Information Division shows cardiology departments in U.S. hospitals are moving beyond separate cardiovascular image management systems (cardiac picture archiving and communications systems [PACS]) and information systems (CVIS), with an eye to the improved efficiency provided by integration with other capabilities such as radiology PACS, other departmental IT systems and the hospital’s EMR (electronic medical records) system.
Transluminal Technologies LLC, a Syracuse, NY-based medical device company, received CE mark approval for the velox CD Vascular Closure Device.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
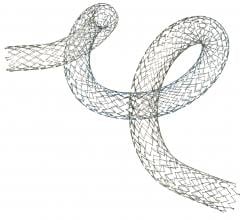
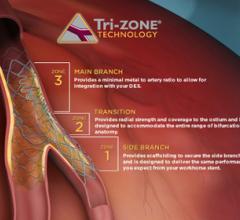
Tryton Medical Inc. announced that the first patient has been enrolled in the EXTENDED Access Registry (Tryton IDE XA registry), a single arm clinical study of its Tryton Side Branch Stent.
August 6, 2014 — St. Jude Medical announced the Center for Medicare and Medicaid Services (CMS) has approved a new technology add-on payment (NTAP) for the CardioMEMS heart failure (HF) monitoring system.
August 6, 2014 — Cerner Corp. and Siemens AG announced the signing of a definitive agreement for Cerner to acquire the assets of Siemens' health information technology business unit, Siemens Health Services, for $1.3 billion in cash.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Acist Medical Systems entered into a strategic agreement with Medtronic to co-promote the world’s first Rapid Exchange FFR (RXi) and high definition IVUS (HDi) technologies in the United States.
Decision Resources Group finds that the United States clot management device market will expand through 2022, particularly in the later years.
August 5, 2014 — A survey, published online in the European Journal of Cardio-Thoracic Surgery, of 13,860 patients who had undergone interventions for aortic valve disease in Germany has revealed that more than 80 percent were in the same or a better state of health one year after the intervention, and was satisfied with the procedural outcome.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

August 5, 2014 — GE Healthcare announced the first integrated, simultaneous, time-of-flight (TOF) capable, whole-body Signa PET/MR (positron emission tomography/magnetic resonance) is 510(k) pending at the U.S. Food and Drug Administration (FDA).

Did you know that medical data on 20,000 people could be exposed to abuse today? According to the U.S. Department of Health and Human Services (HSS), that is the number of people whose protected health information was breached per day on average in 2013. While healthcare practitioners may not realize the value of the data in their care, criminals certainly do. Clinicians and nurses may feel wary of security measures that might slow them down, but there are ways to improve security that will not cost precious moments in an emergency situation. Being lax with security can have a long-lasting impact on all of your patients, not just those with emergencies.
OSTAR Healthcare Technology, a Washington State-based Medical Device and Healthcare IT company, announced the North American launch of its P Series Cloud Based Telehealth Blood Pressure Monitoring System, powered by 3SI (3 simultaneous interpretations) Interpretive Blood Pressure Monitoring Algorithm.

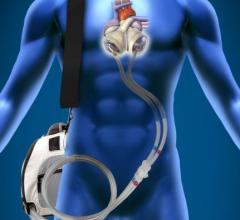
 August 11, 2014
August 11, 2014