Oct. 25, 2025 — Medtronic plc has announced the launch of the Stedi Extra Support guidewire, designed to enhance ...
Oct. 27, 2025 — Elixir Medical, a developer of technologies to treat cardiovascular disease, has announced new clinical ...
Oct. 23, 2025— Emboline, Inc., a provider of full-body embolic protection devices for transcatheter procedures, thas ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
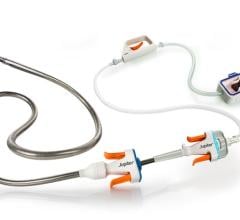
Oct. 26, 2025 – Jupiter Endovascular, Inc., a medical technology company developing a new class of endovascular ...
Oct. 24, 2025 —YorLabs, Inc., a medical technology company developing next-generation intracardiac imaging solutions for ...
Oct. 27, 2025 — The American Heart Association (Association) has launched its latest professional certification program ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Oct. 27, 2025 – Penumbra, Inc. has announced the results of the STORM-PE randomized controlled trial (RCT), which found ...
Oct. 21, 2025 — SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for ...
Oct. 22, 2025 — Heartflow, Inc. has introduced Heartflow PCI Navigator, the newest addition to the Heartflow One ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Oct. 22, 2025 — Qure has announced its latest (510) K clearance from the US Food and Drug Administration (FDA). The ...
Oct. 22, 2025 — Nyra Medical, a leading innovator in structural heart therapies, has announced the initiation of its ...
Oct. 22, 2025 — Basking Biosciences, a clinical-stage biopharmaceutical company developing the first reversible ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Oct. 21, 2025 — Early and accurate detection of heart rhythm problems can mean the difference between life-saving ...
Oct. 21, 2025 – AskBio Inc., a gene therapy company wholly owned and independently operated as a subsidiary of Bayer AG ...
Oct. 21, 2025 — GE HealthCare has received CE mark for its Carevance patient monitor, advancing accessible and reliable ...


 October 28, 2025
October 28, 2025