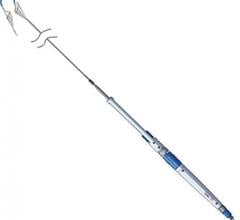
September 11, 2014 — Medtronic announced the U.S. Food and Drug Administration (FDA) 510(k) clearance and launch of the NC Euphora noncompliant balloon dilatation catheter. The new device will be featured for the first time in the United States at the Medtronic booth at the upcoming Transcatheter Cardiovascular Therapeutics (TCT) meeting in Washington, D.C.
Growing awareness of the harmful effects of radiation exposure is driving the uptake of ultrasound systems, which are radiation free, less expensive, and more versatile than bigger modalities such as magnetic resonance (MR).
Geneia announced a multi-year, strategic partnership with Covidien to help improve the health of and reduce the costs for chronically ill patients.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
U.S.-based Medtronic said it would purchase Ireland-based Covidien for $42.9 billion, combining two of the world's biggest medical device makers and helping Medtronic savings with a headquarters change.
Medtronic Inc. announced the first implants in a clinical trial that will compare patient and healthcare system outcomes — including patient mortality and hospitalizations — in heart failure patients who have cardiac resynchronization therapy (CRT) devices with the AdaptivCRT feature enabled versus patients receiving standard CRT.
Only 25 percent of interviewed ambulatory providers report that meaningful use is having a positive impact on patient care.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

September 10, 2014 — According to new research performed at the Mayo Clinic, iodine-based contrast material injected intravenously to enhance computed tomography (CT) images can be safely used in most patients. The study appears online in the journal Radiology.
September 10, 2014 — ResMed announced results from a study presented at the 2014 European Society of Cardiology (ESC) Congress in Barcelona, Spain, looking at the use of the at-home, contactless, bedside SleepMinder device to diagnose sleep-disordered breathing (SDB), the most common co-morbidity in patients with heart failure (HF).
Transcatheter Cardiovascular Therapeutics (TCT) has announced the late-breaking trial presentations at TCT 2014, Sept. 13-17.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 9, 2014 — Tryton Medical Inc. announced an initial closing of an aggregate $20 million private equity financing. Participating in this financing were existing investors RiverVest Venture Partners and 3x5 Special Opportunity Fund, joined by new investor Canepa Advanced Healthcare Fund and an unnamed investor.
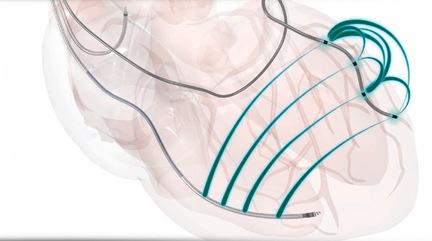
September 9, 2014 — Use of a quadripolar left ventricular (LV) lead instead of a bipolar option during cardiac resynchronization therapy (CRT) can decrease complications at six months, according to preliminary hot line results presented at the 2014 European Society of Cardiology (ESC) Congress in Barcelona.
Claret Medical Inc. vascular and cardiac surgery procedures, announced that it has entered into an agreement for up to $18 million in a Series B financing.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Cook Medical has enrolled the first patient in the clinical study in China of its Zilver PTX Drug Eluting Peripheral Stent. The case was performed by one of the study’s principal investigators, Prof. Peng Liu, M.D., at the China-Japan Friendship Hospital in Beijing.
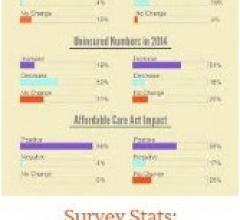
Community Health Centers (CHCs) across the country, which are the medical home for one in 15 people in the United States, are experiencing an overall increase in patients but fewer uninsured patients in 2014, according to a survey conducted by Direct Relief at the National Association of Community Health Centers (NACHC) annual conference last week. ?
September 8, 2014 — Esaote North America announced its new MyLab Six ultrasound system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is now available for sale in the United States.

 September 11, 2014
September 11, 2014