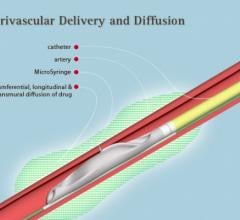
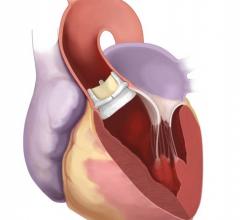
VIVA (Vascular Interventional Advances) Physicians announced a number of highly anticipated late-breaking clinical trial results at VIVA 16, hosted at the Wynn Las Vegas Sept. 18-22.
October 12, 2016 — The Society for Vascular Surgery (SVS) has released new reporting standards focused on endovascular ...
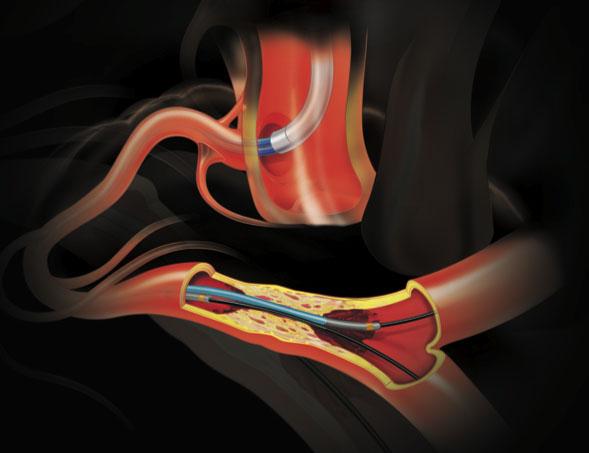
While guidewires are a key tool used by all interventionalists in the cath lab, most operators do not have a deep ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Medtronic plc announced recently that the U.S. Food and Drug Administration (FDA) has cleared the TrailBlazer angled support catheter for use in the peripheral vascular system. Support catheters such as the Trailblazer are often used in endovascular procedures treating complex peripheral artery disease (PAD).
Awards from the National Institutes of Health’s Common Fund are supporting research on the peripheral nervous system, in hopes of finding new ways to treat conditions such as asthma, diabetes and nausea. The new awards total more than $20 million in fiscal year 2016, and go to 27 multidisciplinary research teams through the Stimulating Peripheral Activity to Relieve Conditions (SPARC) program. The SPARC program plans to support awards totaling approximately $238M through fiscal year 2021, pending available funds.
The U.S. Food and Drug Administration (FDA) has issued 510(K) Class II clearance to a web client for the AirStrip One mobile interoperability platform and application. The system can be run on desktops and laptops using Internet Explorer and Google Chrome.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 10, 2016 — ZipLine Medical Inc. recently announced results from a study recently published online in Pacing and ...
Francesco Maisano, clinic director at the University Hospital Zurich, recently led a team of cardiac surgeons and cardiologists in for the first time repairing a leaky tricuspid valve using a new catheter technology.
St. Jude Medical Inc. announced the U.S. clearance and launch of the company’s new PressureWire X Guidewire fractional flow reserve (FFR) measurement system. The latest generation of the PressureWire Guidewire system is designed to offer improved shapeability and better shape retention aimed at reducing vessel trauma, with the accuracy and simplicity physicians expect when treating patients during percutaneous coronary intervention (PCI), especially those with complex anatomies.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

The transradial revolution is one of the fastest growing trends in cardiology. Compared to the femoral access technique ...
Mercator MedSystems recently announced that 13-month data from the DANCE trial was presented during a late-breaking scientific session at the Vascular Interventional Advances (VIVA) Annual Conference 2016. DANCE is a prospective, multicenter, single-arm study designed to assess the clinical performance of the localized delivery of a generic steroid, dexamethasone, to the tissues around arteries that have been injured during endovascular interventions, using Mercator’s proprietary Bullfrog Micro-Infusion Device.
October 7, 2016 — A Northwestern Medicine cardiac surgeon was recently the first in Illinois and second in the United ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 6, 2016 — Xeltis announced this week that patients implanted with its bioabsorbable cardiovascular conduit ...
St. Jude Medical Inc. announced a full market release of its EnSite Precision cardiac mapping system and new Sensor Enabled tools in Europe. The new platform is now installed and active in more than 100 sites across Europe and has been used to support more than 5,000 ablation cases since the system’s CE Mark approval in January 2016.
Interventional labs now have an imaging system that provides clinicians flexibility to perform a wide array of procedures with the launch of Toshiba’s Infinix-i Sky +*. The ceiling-mounted system features a double sliding C-arm and 12 x 16-inch flat panel, offering clinicians the potential to increase coverage, speed and patient access.


 October 12, 2016
October 12, 2016