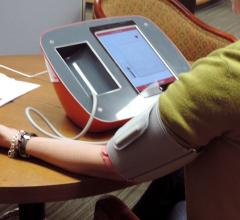
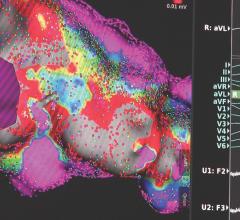
April 13, 2018 — Medical advice about implanted cardiac defibrillators obtained via an online message board appears to ...
Biotronik U.S. and Aziyo announced a strategic agreement allowing Biotronik to distribute Aziyo's CanGaroo extracellular matrix (ECM) cardiovascular implantable electronic device (CIED) envelopes in the United States. BioEnvelope will be available from Biotronik starting in April 2018.
French medical technology company CorWave announced it has been awarded almost $3.5 million in financing for its R&D program NovaPulse in the Silver Economy category. This is part of phase 2 of France’s Concours Mondial d'Innovation or Worldwide Innovation Challenge.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Women who give birth to infants with congenital heart defects may have an increased risk of cardiovascular hospitalizations later in life, according to new research in the American Heart Association’s journal Circulation.
Medtronic plc announced one-year results from the CRYO4PERSISTENT AF study of ablation with the Arctic Front Advance Cryoballoon to isolate the pulmonary veins in patients with symptomatic persistent atrial fibrillation (AF). The Arctic Front Advance Cyroablation System is not approved for treating persistent AF in the United States.
The biggest workplace concern for interventional cardiologists and cath lab staff is their daily exposure to ionizing ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
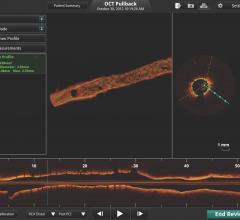
Abbott today announced the initiation of a clinical trial evaluating long-term outcomes of patients who undergo stent implantation guided by optical coherence tomography (OCT) compared to angiography. The trial (ILUMIEN IV) is the first large-scale randomized global study using Abbott's OCT imaging in patients with high-risk, complex coronary artery disease. Patients in the study will be randomized to either OCT-guided or traditional angiography to guide placement of one or more Xience everolimus-eluting coronary stents.
https://www.dicardiology.com/channel/vascular-closure-devicesCardiva Medical announced the company received U.S. Food and Drug Administration (FDA) approval for an expanded indication of the Vascade Vascular Closure System. Already approved for use in arterial closure, Vascade is now FDA-approved for use in 5-7F femoral venous closures as well. This expansion creates an opportunity to better treat patients undergoing interventional cardiac catheterization procedures, while also greatly increasing the available market for the device.
University Hospitals Harrington Heart & Vascular Institute has officially opened the APOLLO trial, implanting the first Intrepid transcatheter mitral valve replacement system in the study. Alan Markowitz, M.D., and Guilherme F. Attizzani, M.D., performed the procedure, the first in the state of Ohio.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Pfizer Inc. announced that the Tafamidis Phase 3 Transthyretin Cardiomyopathy (ATTR-ACT) study met its primary endpoint with a statistically significant reduction in all-cause mortality plus frequency of cardiovascular-related hospitalizations compared to placebo at 30 months. The preliminary safety data showed that tafamidis was generally well tolerated in this population and no new safety signals were identified.1
YMCA of the USA (Y-USA) and the American Heart Association (AHA) are combining efforts to help more people better manage their blood pressure. Beginning this spring, more than 100 clinics participating in the Target: BP initiative will be able to refer qualified patients to enroll at no cost in the YMCA’s Blood Pressure Self-Monitoring program.
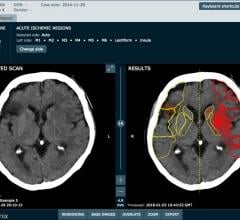
April 5, 2018 — Medical imaging company Brainomix has attracted £7m ($9.8 million) of investment to bring its artificial ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
April 5, 2018 — Diagnostic and Interventional Cardiology (DAIC) magazine received the 2018 Jesse H. Neal Award for Best ...
The Ohio State University Wexner Medical Center will establish the nation’s first center dedicated to treating those with heart failure and arrhythmia with gifts totaling $18 million from Bob and Corrine Frick.
Boston Scientific Corp. announced the acquisition of Securus Medical Group Inc., a privately-held company that has developed a thermal monitoring system for the continuous measurement of esophageal temperature. Boston Scientific has been an investor in Securus since 2016, and the transaction price for the remaining stake not already owned consists of $40 million in cash up-front, as well as up to $10 million in contingent payments based on regulatory achievements and commercial milestones.

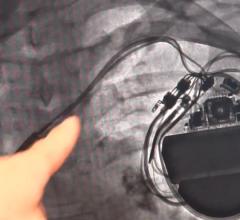
 April 13, 2018
April 13, 2018