August 11, 2016 — Carew Medical Wear Inc., a custom medical outfit company specializing in left ventricular assistance ...
August 11, 2016 — Cardiovascular diseases are the leading cause of death in the United States. With one in every four ...
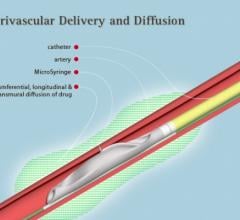
August 10, 2016 — Mercator MedSystems Inc. announced the enrollment of the first critical limb ischemia (CLI) patients ...
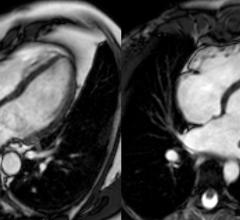
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
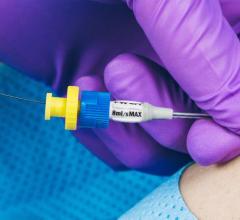
August 10, 2016 — Access Scientific introduced the PowerWand XL, a companion to the PowerWand All-in-One, in July. The ...
Scientists have shown that people who exercise for even a few hours each week can enlarge their hearts. This is a normal and beneficial response to exercise, but until now has only been recognized in athletes. The researchers say that doctors should now consider an individual’s activity level before diagnosing common heart conditions.

In the past few years, concern has skyrocketed from interventional cardiologists and cath lab staff over radiation dose ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
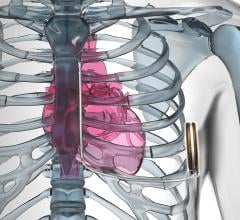
August 9, 2016 — The U.S. Food and Drug Administration (FDA) granted market approval for the Emblem MRI Subcutaneous ...
August 9, 2016 – With the use of intensive care units (ICUs) on the rise in many hospitals, researchers at LA BioMed and ...
Updated recommendations from the American Academy of Neurology (AAN) states that catheter-based closure should not be routinely recommended for people who have had a stroke and also have a heart defect called a patent foramen ovale (PFO), a channel between the top two chambers in the heart.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
ClearFlow Inc. announced in late July that its PleuraFlow Active Clearance Technology is now available for the treatment of pediatric cardiothoracic surgery patients.
August 5, 2016 — The Centers for Medicare and Medicaid Services (CMS) has reassigned MitraClip transcatheter mitral ...
August 5, 2016 — Osprey Medical Inc. said it received commitments from investors for approximately $28 million ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology (DAIC) ...

August 4, 2016 — Medtronic announced CE (Conformité Européenne) mark market clearnace for the self-expanding ...
As more procedures move into the cath lab, growing complexity is fueling an increase in procedural volume and the need ...


 August 11, 2016
August 11, 2016