Imricor Medical Systems announced enrollment of the first patients in a clinical study to evaluate the Vision-MR Ablation Catheter for the treatment of atrial flutter under real-time magnetic resonance imaging (MRI) guidance.
May 10, 2016 — Cardiovascular magnetic resonance (CMR) is a stronger predictor of risk for major adverse cardiovascular ...
Medtronic plc announced clinical results highlighting the strong safety and performance profile of the miniaturized Micra Transcatheter Pacing System (TPS) at Heart Rhythm 2016, the Heart Rhythm Society's 37th Annual Scientific Sessions, May 4-7 in San Francisco.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The first-ever trial using new SonR hemodynamic sensor technology proves to be safely and effectively applied in cardiac resynchronization therapy (CRT) patients.
Results from a first-of-its-kind study identify a significant increase in the incidence of atrial fibrillation (AF) among patients with pacemaker-detected sleep apnea.
A new study shows the use of novel anticoagulants to treat atrial fibrillation (AF) on an “as needed basis” guided by diligent pulse monitoring can be an effective and safe way to lower overall risk of stroke.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 9, 2016 — New data presented at the Heart Rhythm Society's (HRS) annual meeting highlighted the long-term safety of ...
A new study has found an increased risk of dementia in patients with atrial fibrillation (AF) that receive long-term blood thinner warfarin (Coumadin) compared to patients that use warfarin for conditions other than AF.
May 9, 2016 —St. Jude Medical announced presentation of the MultiPoint Pacing (MPP) Investigational device exemption ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 9, 2016 — A new study presented at the Heart Rhythm Society’s 37th Annual Scientific Sessions, found that among ...
May 9, 2016 — Medtronic announced the results of several studies evaluating a novel approach to implantable cardioverter ...
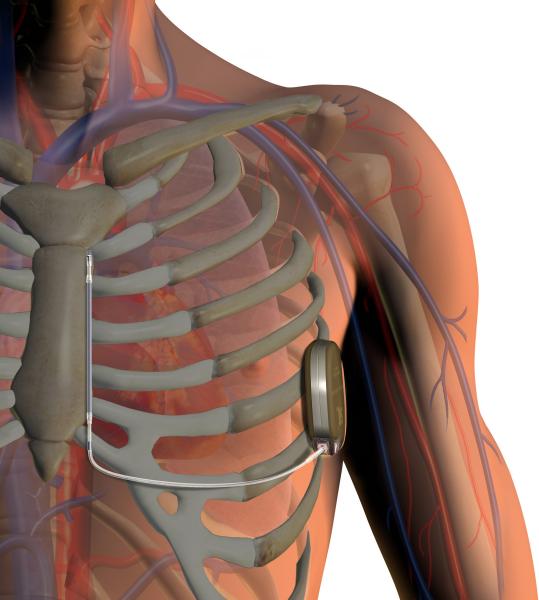
May 9, 2016 — The mid to long-term results from the EFFORTLESS study, the largest subcutaneous implantable cardioverter ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 9, 2016 – A new, large study shows that 95.7 percent of individual leads were successfully removed using a procedure ...
Atrial fibrillation patients taking warfarin are at higher risk of developing kidney failure if anticoagulation levels are not properly managed, according to a new study from researchers at the Intermountain Medical Center Heart Institute.
The largest risk-directed study by a national hospital system demonstrates a 40 percent decline in bleeding events for percutaneous coronary intervention (PCI) patients and a significant reduction in pharmacy costs.


 May 10, 2016
May 10, 2016