Lithoplasty is a balloon-based technology that utilizes integrated lithotripsy to disrupt both superficial and deep calcium and normalize vessel wall compliance prior to low-pressure balloon dilatation.
ECRI Institute’s 2015 Top 10 Hospital C-Suite Watch List, available as a free public service, answers key questions on new and emerging health technologies that potentially provide new ways to treat patients, improve care and reduce costs.
CardioFocus Inc., developer of the HeartLight Endoscopic Ablation System for the treatment of atrial fibrillation (AF), announced that it has executed an exclusive, multi-year distribution agreement with Japan Lifeline Co. Ltd.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Transcatheter Technologies GmbH has announced that it is expanding its product family to include Tresillo, a transcatheter mitral valve implantation (TMVI) version.
Medtronic, Inc. announced the initiation of a randomized clinical trial in Europe to assess the benefits of ablation using the Medtronic Artic Front Advance cryoballoon as a first-line treatment for atrial fibrillation (AF) patients.
W. L. Gore & Associates Inc. announced that the Gore Viabahn Endoprosthesis has received CE Mark approval to improve blood flow in symptomatic obstruction of peripheral veins, excluding the venae cavae and pulmonary veins.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
CardioKinetix Inc. announced results of a pooled analysis study of the catheter-based Parachute ventricular partitioning device. Twelve-month clinical data from PARACHUTE III, a study of 100 post-market European patients with ischemic heart failure treated consecutively between 2011 and 2013, were presented at the 2014 TCT Conference in Washington, D.C., and the 2014 HFSA Conference in Las Vegas.
The Healthcare Colloquium announced that CHI Franciscan Health is the first Accredited Heart Failure System in the Northwest.
With today’s aging population in the United States, we are experiencing an increased occurrence of cardiovascular disease including heart failure, degenerative valve disease and atrial fibrillation. These clinical challenges, along with changes in healthcare economics and standards, require efficient, effective and streamlined diagnostic solutions. These solutions must deliver critical added benefits including:
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Covidien announced it has received CE Mark approval for its Stellarex drug-coated angioplasty balloon (DCB).
At the IEEE medical Imaging Conference (MIC) in November, ContextVision co-presented with Texas Instruments and High Performance Consulting on new research on 3-D adaptive filtering.
Medtronic's roughly $43 billion acquisition and merger with Covidien has been widely discussed in the media for its U.S. Federal tax implications, but it's also creating a stronger leading player in one of the fastest growing device markets, according to Kalorama Information.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Stentys announced that the Self-Apposing Stent has been implanted more than 10,000 times in patients worldwide
Research and Markets has announced the addition of the "MediPoint: Nuclear Imaging - PET and SPECT Equipment - Global Analysis and Market Forecasts" report to their offering
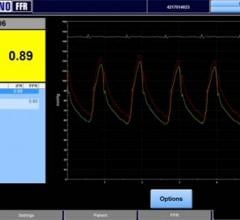
Volcano Corp. announced that more than 1,000 systems have been activated with its instant wave-Free Ratio (iFR) Modality software, featuring a simplified workflow and reduced need for hyperemic agents.

 January 13, 2015
January 13, 2015