Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign ...
Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new ...
Jan. 31, 2026 — The Society of Thoracic Surgeons (STS) has elected Vinay Badhwar, MD, as its 62nd president during the ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Feb. 2, 2026 — GE HealthCare has announced that Allia Moveo has received U.S. Food and Drug Administration (FDA) 510(k) ...
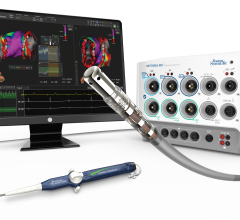
Jan. 28, 2026 — Corify Care has announced a major development in cardiac electrophysiology with the publication of its ...
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Jan. 27. 2026 — Circle Cardiovascular Imaging Inc. has announced the release of cvi42 v6.4, the latest version of its ...
Jan. 27, 2026 — BURL Concepts, a private company developing a rapid, automated and affordable test for evaluating ...
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
Jan. 27, 2026 — A new national study reveals a stark disconnect between Americans’ desire for preventive cardiac ...
Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic ...
Jan. 26, 2026 — AISAP, a provider of AI point-of-care diagnostics, has published a new clinical study in the peer ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
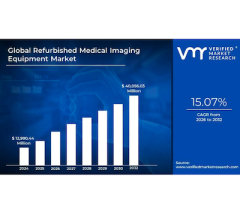
Jan. 11, 2026 — The Global Refurbished Medical Imaging Equipment Market Size is projected to grow at a CAGR of 15.07% ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 20, 2026 — Kardium Inc. has announced the publication of the PULSAR clinical trial results in the Journal of the ...


 February 03, 2026
February 03, 2026