AVT, a new advanced visualization solution from Barco, reportedly answers the demand for instant access to 3D anywhere, anytime. With AVT, doctors can read volumetric studies throughout the hospital, in a remote office or even at home.
ARJO offers a full line of sliding surfaces including bariatric Maxi Slide sheets, for bed boosting and lateral ...
February 21, 2008 - Accumetrics Inc. has raised $28.8 million in its Series D financing, which will enable the company ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

There are several patient warming and cooling devices available for temperature management, but the practice of induced ...
Baxter Healthcare Corp. received FDA 510(k) clearance for its needleless V-Link Luer-activated device (LAD), an IV ...
Power Medical Interventions Inc. said the FDA cleared its intelligent iDrive surgical devices, which includes a suite of ...
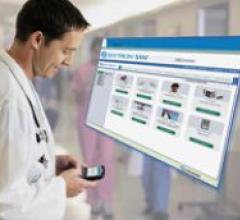
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The SafeSeal Hemostasis Patch topical wound dressing is designed to decrease the time it takes to control bleeding from the puncture made into a blood vessel to perform an endovascular procedure.
To assist facilities with the transfer of patient images and/or medical records into an electronic information management system, eHealth Global Technologies will introduce its new service at HIMSS for migrating film or paper into a digital format.
Physio-Control Inc., a wholly owned subsidiary of Medtronic Inc., offers the LUCAS Chest Compression System in the U.S. through an exclusive distribution partnership with JOLIFE AB, expanding the reach of its emergency medicine device portfolio.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Heavy duty, yet lightweight, the Detecto 6856 bariatric scale’s balanced design reportedly makes it easy to move ...
February 21, 2008 - High schools and middle schools in Long Branch, NJ, are using a computer-assisted medical device ...

As hospitals migrate to digital imaging and picture archive and communications systems (PACS) and video become important ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
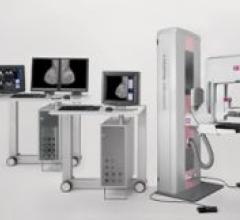
Siemens Healthcare MAMMOMAT Novation S was created to help detect, diagnose and treat breast cancer earlier, faster and ...
Skytron�s new patented wireless RFID Asset Tracking Manager, powered by Awarepoint, is specifically designed for ...
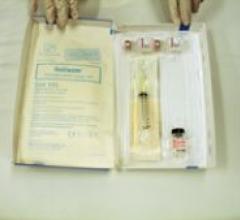
Baxter Healthcare Corp. received FDA approval in December for the GELFOAM Plus Hemostasis Kit (absorbable gelatin sponge ...

 February 20, 2008
February 20, 2008