Medtronic and the U.S. Food and Drug Administration (FDA) announced a Class I Urgent Medical Device Recall of the MindFrame Capture LP revascularization device on account of a risk of the delivery wire breaking or separating during use.
May 21, 2018 — Cardiology medical device reprocessing company Innovative Health recently received U.S. Food and Drug ...
May 21, 2018 — A recent study published in Heart and Vessels has found that the use of the Baylis Medical NRG ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
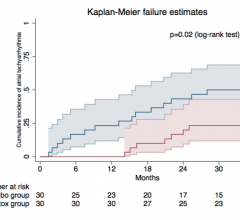
Data on the effectiveness of cardiac resynchronization therapy (CRT) in patients with non-left bundle branch block (non-LBBB) is limited and when available has been shown to be suboptimal compared to LBBB patients. A new study presented at the 2018 Heart Rhythm Society (HRS) Scientific Sessions, May 9-12 in Boston, compared the effects of targeting the region of increased electrical delay (QLV approach) for left ventricular (LV) lead location to a standard of care (SOC) anatomical implant approach in non-LBBB patients. The comparison was assessed on the Clinical Composite Score (CCS) after 12 months of follow-up.
In a new study, cardiac contractility modulation (CCM) therapy was confirmed to significantly improve exercise tolerance (ET) and quality of life (QoL) for patients with persistent symptomatic heart failure and an ejection fraction between 25 and 45 percent. Results of the prospective FIX-HF-5C Study were presented at the 2018 Heart Rhythm Society (HRS) Scientific Sessions, May 9-12 in Boston.
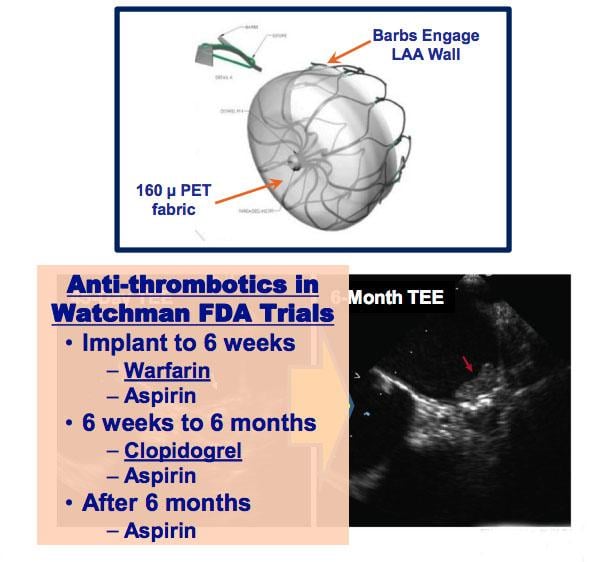
May 18, 2018 — Left atrial appendage closure (LAAC) with the transcatheter Watchman device prevents thromboembolism from ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
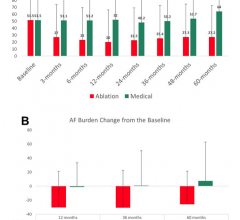
May 18, 2018 — Catheter ablation for atrial fibrillation (AF) in patients with heart failure in the CASTLE-AF Trial was ...
May 18, 2018 — Nearly half of patients prescribed warfarin and just under one third of those using newer direct oral ...
May 18, 2018 — A significant number of patients with symptomatic premature ventricular contractions (PVCs) have ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 18, 2018 — Three year results of a study found injection of botulinum toxin into epicardial fat pads in patients ...
Here is an aggregation of all the news and late-breaking studies presented at the 2018 Heart Rhythm Society (HRS) ...
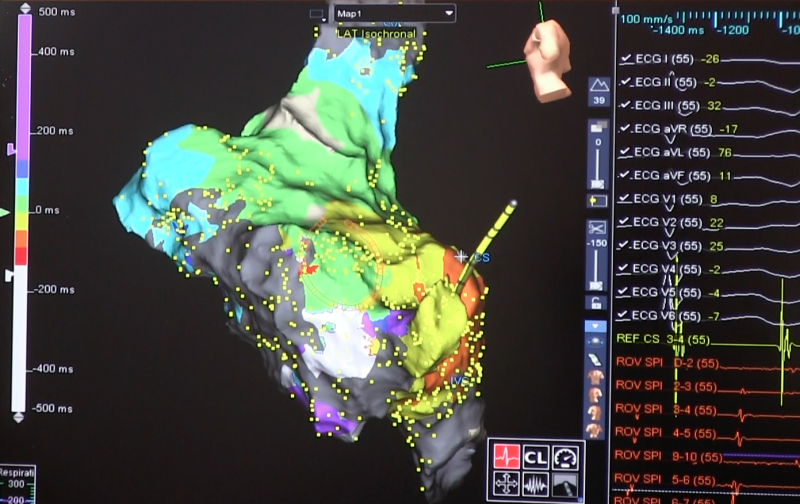
May 16, 2018 – The first results of the randomized, multicenter, long-term, international CABANA clinical trial were ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
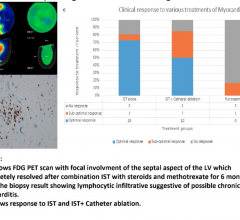
May 10, 2018 – A new study is the first to test the clinical effectiveness of incremental peri-operative antibiotics as ...
Medical device manufacturer CardioFocus Inc. announced a successful live case featuring its HeartLight X3 System during the annual Prague Workshop on Catheter Ablation, April 21-24 in Prague, Czech Republic.
A new study published in JAMA Cardiology used the Zio continuous cardiac monitoring system by iRhythm to provide a comprehensive picture of the burden of atrial fibrillation (AF) in patients. Utilizing this data in combination with electronic health record data, the researchers concluded that an increase in AF burden is independently associated with a higher risk of ischemic stroke and arterial thromboembolism in patients who are not taking anticoagulant medication.


 May 21, 2018
May 21, 2018