August 11, 2014 — NeoCoil LLC announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its new stand-alone wireless headphones and patient alert system. The NeoCoil wireless two-way audio system is NeoCoil's first wireless patient communication and entertainment system designed specifically to work in the magnetic resonance imaging (MRI) environment.
Imaging experts from the American Society of Echocardiography (ASE) released a paper, "Guidelines for the Cardiac Sonographer in the Performance of Contrast Echocardiography: A Focused Update from the American Society of Echocardiography,” to help clinicians optimize and demystify the use of ultrasound contrast media.
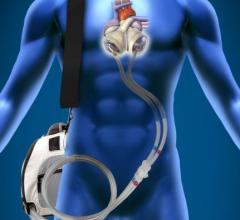
SynCardia Systems Inc. received approval in July from the U.S. Federal Drug Administration (FDA) for the SynCardia temporary Total Artificial Heart with SynHall valves, giving the company control over the last key component to manufacture the Total Artificial Heart
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Running for only a few minutes a day or at slow speeds may significantly reduce a person’s risk of death from cardiovascular disease compared to someone who does not run, according to a clinical study published in the Journal of the American College of Cardiology.
Biotronik announced the completion of patient enrollment in the superficial femoral artery (SFA) arm of its BIOFLEX-I clinical trial, an U.S. Food and Drug Administration (FDA)-approved investigative device exemption (IDE) trial evaluating the use of self-expanding nitinol stents in treating peripheral artery disease.
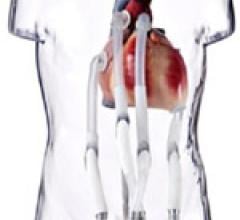
August 7, 2014 — The Berlin Heart Group announced they have completed enrollment in their post-approval study, the only condition of the humanitarian device exemption (HDE) approval that Berlin Heart received for the Excor pediatric ventricular assist device (VAD) on Dec. 16, 2011.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

August 7, 2014 — A newly released study by IMV Medical Information Division shows cardiology departments in U.S. hospitals are moving beyond separate cardiovascular image management systems (cardiac picture archiving and communications systems [PACS]) and information systems (CVIS), with an eye to the improved efficiency provided by integration with other capabilities such as radiology PACS, other departmental IT systems and the hospital’s EMR (electronic medical records) system.
Transluminal Technologies LLC, a Syracuse, NY-based medical device company, received CE mark approval for the velox CD Vascular Closure Device.
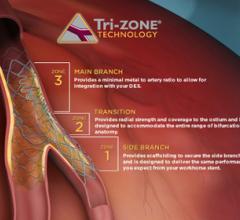
Tryton Medical Inc. announced that the first patient has been enrolled in the EXTENDED Access Registry (Tryton IDE XA registry), a single arm clinical study of its Tryton Side Branch Stent.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
August 6, 2014 — St. Jude Medical announced the Center for Medicare and Medicaid Services (CMS) has approved a new technology add-on payment (NTAP) for the CardioMEMS heart failure (HF) monitoring system.
August 6, 2014 — Cerner Corp. and Siemens AG announced the signing of a definitive agreement for Cerner to acquire the assets of Siemens' health information technology business unit, Siemens Health Services, for $1.3 billion in cash.

Acist Medical Systems entered into a strategic agreement with Medtronic to co-promote the world’s first Rapid Exchange FFR (RXi) and high definition IVUS (HDi) technologies in the United States.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Decision Resources Group finds that the United States clot management device market will expand through 2022, particularly in the later years.
August 5, 2014 — A survey, published online in the European Journal of Cardio-Thoracic Surgery, of 13,860 patients who had undergone interventions for aortic valve disease in Germany has revealed that more than 80 percent were in the same or a better state of health one year after the intervention, and was satisfied with the procedural outcome.

August 5, 2014 — GE Healthcare announced the first integrated, simultaneous, time-of-flight (TOF) capable, whole-body Signa PET/MR (positron emission tomography/magnetic resonance) is 510(k) pending at the U.S. Food and Drug Administration (FDA).

 August 11, 2014
August 11, 2014