May 23, 2011 - Doctors in Germany are among the first to treat ischemic stroke patients outside the United States with the Revive SE, a new self-expanding blood clot retrieval and removal device designed to remove blood clots and restore blood flow to the brain in patients having a stroke.
May 23, 2011 -- Boston Scientific Corporation announced results from a clinical study evaluating the use of its Watchman Left Atrial Appendage Closure Device in patients with atrial fibrillation who have a contraindication to oral anticoagulants such as warfarin. Data were presented at the annual EuroPCR Scientific Program in Paris by Martin Bergmann, M.D., Department of Cardiology at the Asklepios Klinik St. Georg in Hamburg, Germany, and principal Iinvestigator of the study.
May 20, 2011 -- Medrad Inc., a business of Bayer HealthCare, has announced the launch of the next generation Intego PET Infusion System, which features a new design that is 38 percent smaller, power-driven and includes enhancements to technologist workflow. The new Intego will be on display at the Annual Meeting of the Society of Nuclear Medicine (SNM).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 20, 2011 - Boston Scientific Corporation today announced 12-month results from its PLATINUM Small Vessel study, demonstrating excellent safety and effectiveness outcomes for the 2.25mm Promus Element Everolimus-Eluting Platinum Chromium Stent System in treating small vessel coronary disease. The study is a global, prospective, single-arm, subtrial of the PLATINUM clinical program. It compares the Promus Element Stent (2.25mm) in 94 patients with small vessels (greater than or equal to 2.25 to less than 2.50 mm reference vessel diameter and less than or equal to 28mm lesion length) to a pre-specified performance goal based on results from patients treated with the Taxus Express Paclitaxel-Eluting Stent.
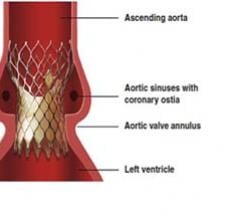
May 20, 2011 - Edwards Lifesciences Corporation announced that clinicians continued to achieve successful one-year outcomes in high-risk or inoperable patients undergoing transcatheter aortic valve replacement during the first two years of commercialization of the Edwards Sapien valve. Data on the more than 2,300 patients enrolled in the post-market European SOURCE Registry since November 2007 were presented in a late-breaking clinical trial session today at EuroPCR 2011.
May 20, 2011 – e-BioMatrix post-marketing surveillance (PMS) registry information presented at EuroPCR 2011 has confirmed that BioMatrix, Biosensors’ Biolimus A9-eluting stent system with abluminal bioresorbable polymer, is safe over a 12-month period in a “real-world” patient population.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
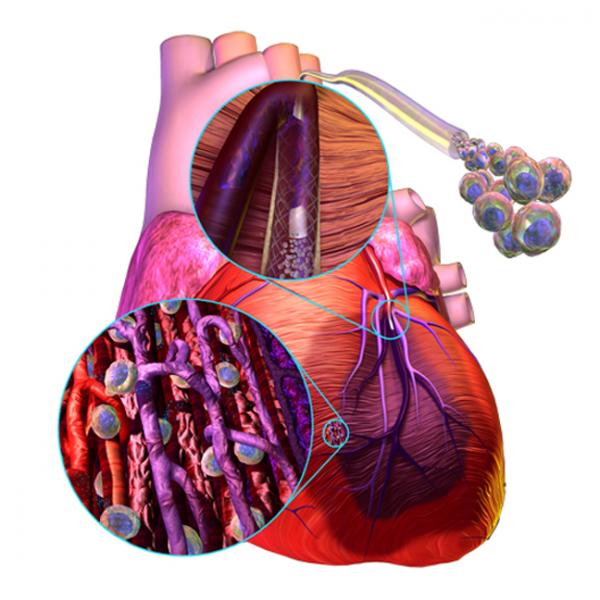
For years researchers have experimented with stem cells in attempts to regrow myocardium in patients who suffer permanent tissue damage from myocardial infarction. A handful of companies are pushing forward with clinical trials of their technologies in an effort to show this futuristic concept has promise.
Over the last decade, cardiac stem cell therapy has shown tantalizing but as yet unrealized potential as a treatment for the left-ventricular dysfunction (LVD) that develops subsequent to myocardial infarction (MI). Experimentally, somatic stem cells of various lineages, as well as pluripotent stem cells, have been induced to transform into contractile myocytes expressing cardiac-specific proteins.
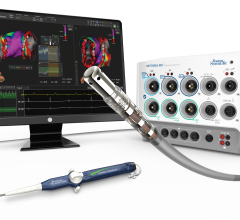
Only a few years ago, separate software systems without a common interface were the industry standard to manage electrocardiograms (ECGs), hemodynamic monitoring systems in the cath lab, picture archiving and communications systems (PACS), electrophysiology, cardiac ultrasound and other areas of cardiac specialty.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

For decades, cath lab clinicians essentially had only one option for achieving hemostasis management following a procedure – compression. That changed in the 1990s with the introduction of vascular closure devices and hemostatic pads, which were designed to significantly shorten the time it took to close the wound left at the access site.

According to the American College of Cardiology (ACC), each year nearly 400,000 patients are admitted to U.S. hospitals with ST segment elevation myocardial infarction (STEMI). National ACC/American Heart Association (AHA) guidelines state that hospitals treating STEMI patients with emergency percutaneous coronary interventions (PCI) should reliably achieve a door-to-balloon time (D2B) of 90 minutes or less, and studies have demonstrated strong associations between time to primary PCI and in-hospital mortality risk.
May 19, 2011—Arterial Remodeling Technologies (ART) announced details of the state-of-the-art design of its polymer-based bioresorbable stent platform at EuroPCR 2011.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 19, 2011 – New clinical data presented today at EuroPCR 2011 demonstrate immediate and long-term health-related quality-of-life (HRQoL) benefits for patients receiving the Medtronic CoreValve System from Medtronic Inc. The favorable two-year results from the French CoreValve multicenter prospective study are the largest set of CoreValve HRQoL data and the longest-term HRQoL data available from all transcatheter aortic valve implantation (TAVI) systems.
May 19, 2011 - Neovasc Inc., a developer of novel technologies used to treat vascular disease, reported positive six-month follow-up data for a patient with severe refractory angina who received the Neovasc Reducer product in a “live case” procedure broadcast during the 22nd annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in September 2010. The data, which showed a marked improvement in the patient’s angina symptoms, was presented today at EuroPCR, a leading European cardiovascular conference.
May 19, 2011 - Results from a study validating Cheetah Medical's noninvasive NICOM system specifically for use in patients with pulmonary hypertension were presented Wednesday, May 18th, at the 2011 American Thoracic Society Annual Meeting in Denver.

 May 23, 2011
May 23, 2011