Feature | February 06, 2014 | Dave Fornell
New EP Mapping Systems Enter the U.S. Market
Systems offer Improved Speed and Accuracy
New electrophysiology (EP) ablation mapping/navigation systems recently entered the U.S. market, each offering technology the vendors say will speed procedure time and improve procedural accuracy.
Boston Scientific’s Rhythmia Mapping System, a 3-D mapping and navigation system, gained U.S. Food and Drug Administration (FDA) 510(k) clearance in 2013. It is designed to intelligently automate map creation, increase speed and improve the density of mapping, capturing thousands versus hundreds of data points. The system also features vMap, a validation map, which is designed to enable EPs to rapidly confirm the endpoints of the ablation treatment. The intelligence built into the system also helps eliminate the need for manual annotation.
The Rhythmia mapping system works with Boston Scientific’s 64-electrode IntellaMap Orion high-resolution mapping catheter.
FIRM Rotor Mapping
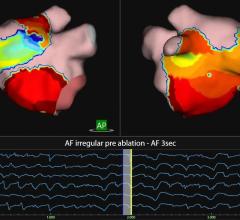
Atrial fibrillation has proved to be an incredibly challenging arrhythmia to treat, and its complexity has defied interpretation and visualization by traditional EP mapping approaches. A relatively new technique has been developed using basket-mapping catheters to directly image myocardial bio-electrical rotors, an EP phenomenon shown in clinical studies to sustain atrial fibrillation.
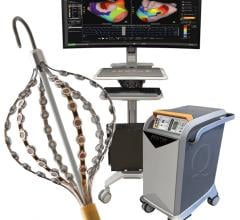
Topera Inc. received 510(k) clearance in December for the latest generation of its 3D Mapping System. The new system has faster processing times, providing near-instantaneous intra-procedural mapping and re-mapping capabilities. The system incorporates a new color-imaging module to aid identification of rotors, an EP phenomenon previously shown to sustain atrial fibrillation. These functionalities enable EPs to more efficiently diagnose and ablate the cause of these complex arrhythmias.
Topera also gained approval for its FIRMap panoramic contactmapping basket catheter in the United States and Europe in late 2013. FIRMap is the first and only contact-mapping basket catheter cleared by the FDA for use in all cardiac chambers to assist in the diagnosis of complex arrhythmias.


 September 05, 2025
September 05, 2025