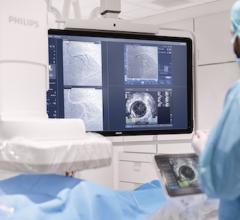
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
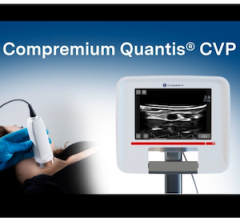
Jan. 8, 2026 — Compremium AG recently announced that the U.S. Food and Drug Administration (FDA) has granted ...
Jan. 12, 2026 — YorLabs, Inc., a medical technology company providing next-generation intracardiac imaging solutions for ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
Jan. 12, 202 — Abbott and AtaCor Medical have announced a collaboration to advance a next-generation investigational ...
Jan. 6, 2026 — Millions of Americans living with heart failure are not receiving medications that have been proven for ...
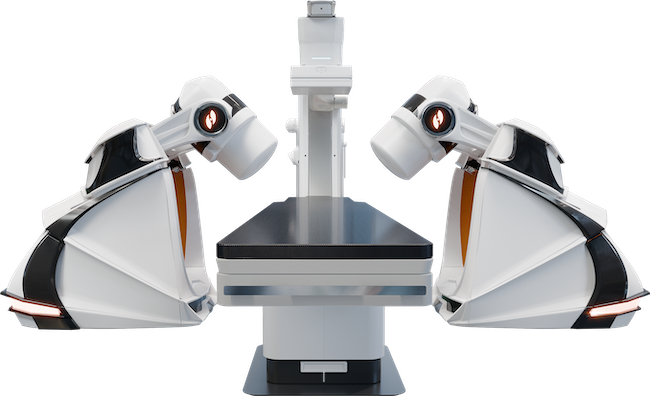
Robotic Magnetic Navigation (RMN) emerged two decades ago as an alternative approach to performing complex ablation ...
Jan. 9, 2026 — Effective January 1, 2026, Elucid's Plaque-IQ coronary plaque analysis has received a new Category I ...
Jan. 7, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has partnered with Jefferson Health, one ...
Dec. 10, 2025 — Medtronic plc has announced the first commercial use of the Liberant thrombectomy system (Liberant) ...
Jan. 6, 2026 — W. L. Gore & Associates’ medical business (Gore) has announced the FDA approval of the Gore Viabahn ...
Dec. 26, 2025 — Edwards Lifesciences has announced the company’s SAPIEN M3 mitral valve replacement system, a ...
Dec. 16, 2025 — A new study, published in the American Journal of Cardiology, found that the use of Computer Assisted ...
Dec. 18, 2025 — Abbott has received U.S. Food and Drug Administration (FDA) clearance and CE Mark for its Amplatzer ...
Dec. 18, 2025 – Ventric Health, a medtech company enabling early detection of heart failure (HF) in a primary care ...
Dec. 15, 2025 — Royal Philips has entered into an agreement to acquire SpectraWAVE, Inc., an innovator in enhanced ...


 January 19, 2026
January 19, 2026