October 29, 2012 — Brainlab released Buzz Digital OR, which is designed to aid information integration for the surgical ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 26, 2012 — Covidien announced final 12-month results from its DEFINITIVE LE (Determination of Effectiveness of ...
October 24, 2012 — Avinger Inc., a medical device manufacturer of multi-functional catheters for crossing CTOs in ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
October 23, 2012 — The use of stents for arterial blockages in the leg has been associated with high rates of late ...
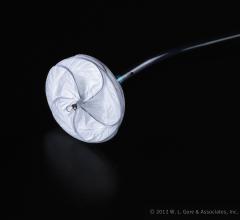
October 22, 2012 — W. L. Gore & Associates announced that the U.S. Food and Drug Administration (FDA) has approved the ...

October 19, 2012 — The 24th Transcatheter Cardiovascular Therapeutics (TCT) conference, sponsored by the Cardiovascular ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 19, 2012 — Elixir Medical Corporation, a developer of products that combine state-of-the-art medical devices ...
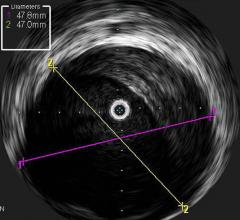
October 19, 2012 — Volcano Corp., a developer and manufacturer of precision-guided therapy tools designed to enhance the ...
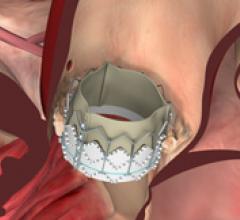
October 19, 2012 — Edwards Lifesciences announced today it received approval from the United States Food and Drug ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
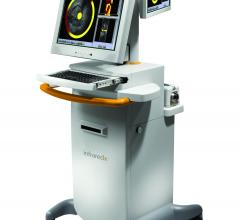
October 18, 2012 – Infraredx Inc. and Royal Philips Electronics announced they have signed a joint development and ...
October 17, 2012 — AngioDynamics entered into a definitive agreement to acquire all the outstanding capital stock of ...
October 16, 2012 — Proteon Therapeutics Inc. has initiated enrollment in a Phase 1 clinical study of its lead product ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 15, 2012 — Three-year data from the Zilver PTX randomized controlled trial of paclitaxel-eluting stents for ...
October 15, 2012 — Surefire Medical Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market ...

October 12, 2012 — Boston Scientific announced it has enrolled the first patient in the REPRISE II clinical trial to ...


 October 29, 2012
October 29, 2012