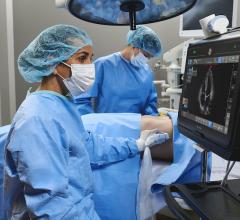
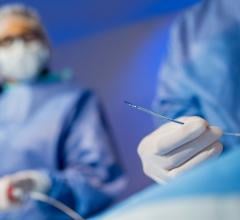
This is a quick example of clinical use of the Shockwave Medical Intravascular Lithotripsy system that uses sonic wave ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
March 31, 2021 — Driven by pandemic realities and clinical demand for portable and intelligent point-of-care ultrasound ...
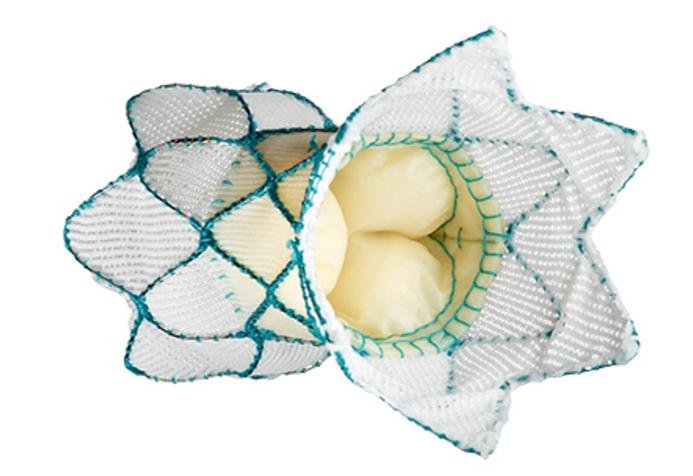
March 26, 2021 — Today, the U.S. Food and Drug Administration (FDA) cleared the Medtronic Harmony Transcatheter ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
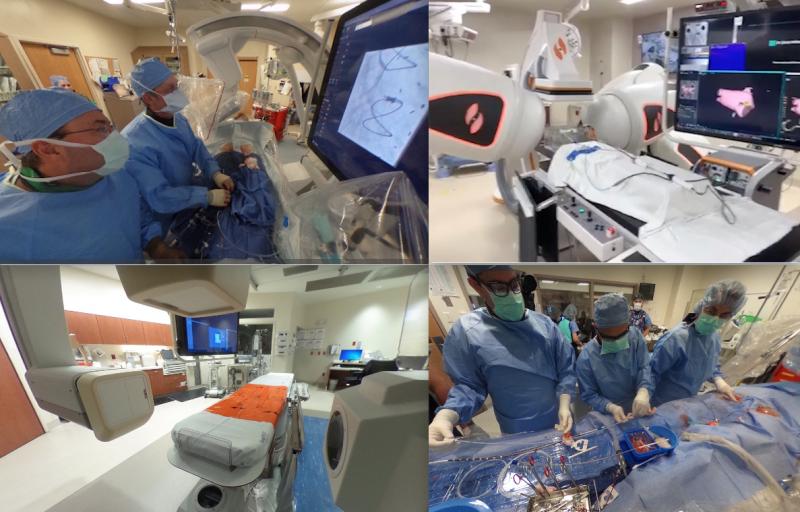
There is a lot of interest in how other hospitals organize and equip their cardiac cath labs and electrophysiology (EP) ...
March 24, 2021 — Endotronix Inc., a digital health and medical technology company working on advancements in treating of ...
Interview with Scott E. Kasner, M.D., who served as the principle investigator for the Gore Cardioform REDUCE trial, and ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
A comprehensive review or more than 80 randomized controlled trials (RCTs) investigating how to best manage optimal ...
March 18, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific's TheraSphere Y-90 Glass ...
After covering cardiovascular technologies for 14 years, it is rare that I find a new technology that might actually be ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

The first devices developed for interventional cardiology were percutaneous transluminal coronary angioplasty (PTCA) ...
March 12, 2021 — Philips Healthcare announced the final, five-year results of two major randomized controlled trials ...
March 12, 2021 — Cardinal Health today announced that it is selling its Cordis cardiology and endovascular business to ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

Intra-cardiac Echocardiography (ICE) uses catheter-based cardiac ultrasound array to image anatomy and devices inside ...

While many cardiac and vascular procedures have largely moved to minimally invasive techniques, the size of these ...

The latest cardiology practice-changing scientific breakthrough, late-breaking study presentations have been announced ...


 March 31, 2021
March 31, 2021