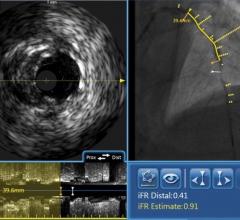
September 25, 2018 – New data demonstrate the correlation between the presence of non-flow-limiting, non-intervened-upon ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
September 25, 2018 — Orchestra BioMed Inc. announced the three-year clinical results from its Sirolimus Angioplasty ...
September 24, 2018 — Ancora Heart Inc. announced positive clinical data from the company’s recently expanded U.S. early ...
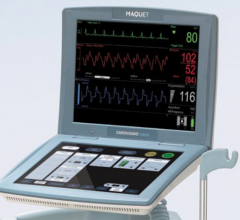
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
September 24, 2018 — New data announced at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21 ...
September 24, 2018 — Corindus Vascular Robotics Inc. announced the first live transmission of a remote interventional ...
September 24, 2018 — Getinge is voluntarily initiating a worldwide recall involving a field correction of approximately ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 21, 2018 — Osprey Medical announced a collaboration with GE Healthcare on Osprey’s Be Kind to Kidneys campaign ...
September 21, 2018 — At the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21-25 in San Diego ...

October 4, 2018 – The Cardiovascular Research Foundation (CRF) had 15 late-breaking trials and 12 late-breaking clinical ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 20, 2018 — At the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting, Sept. 21–25 in San Diego ...
September 19, 2018 — Abiomed Inc. announces its initiatives at the 30th Transcatheter Cardiovascular Therapeutics (TCT) ...
September 19, 2018 – The U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
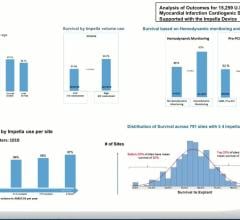
William O’Neill, M.D., outlines his recent clinical publication of AMICS patients from the Impella Quality (IQ) database ...
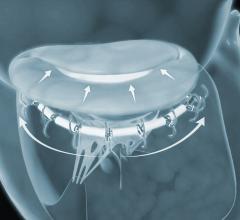
September 17, 2018 — 4C Medical Technologies Inc. announced that its AltaValve transcatheter mitral valve replacement ...
This is an animation showing how the dedicated bifurcation stent developed by Advanced Bifurcation Systems (ABS) is ...


 September 25, 2018
September 25, 2018