June 23, 2020 — Philips announced the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) for ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
June 23, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Program status for the ...
Prior to January 2020 when clinicians read about the history of the 1918 flu, and epidemiologists predicted we were ...
June 19, 2020 — SMT (Sahajanand Medical Technologies Pvt. Ltd) said it acquired of the structural heart medical device ...

June 15, 2020 — The U.S. Food and Drug Administration (FDA) today revoked the emergency use authorization (EUA) that ...
Jay Mohan, D.O., RPVI, interventional cardiology fellow at William Beaumont Hospital, Royal Oak, Michigan, created this ...

(UPDATED information added to this article June 10, 2020)
April 28, 2020 — Medical conferences are where the latest ...
June 10, 2020 — In a new study, Johns Hopkins researchers found that testing people for SARS-CoV-2 (COVID-19) too early ...

June 9, 2020 — SMT (Sahajanand Medical Technology Private Ltd.), a leading medical device company in India focused on ...
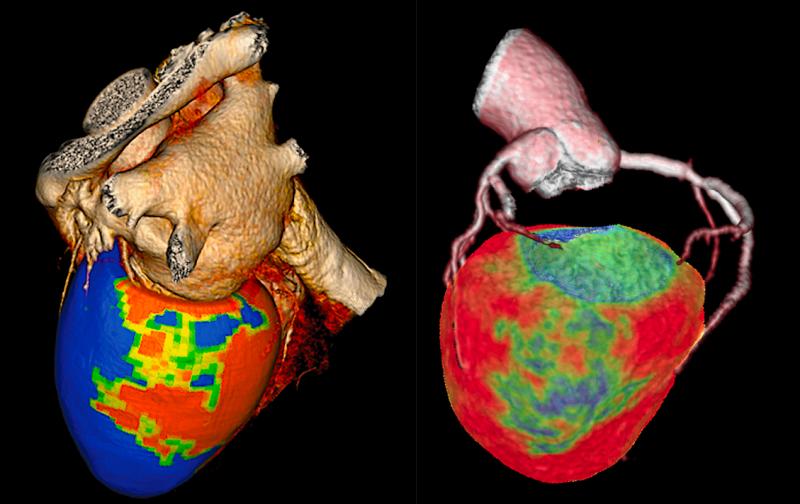
Here is an overview of a few of the biggest technology advances in cardiovascular computed tomography (CT). These are ...
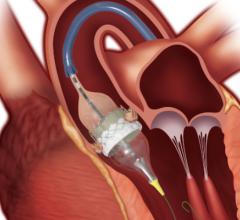
June 8, 2020 — Edwards Lifesciences Corp. announced Chinese regulatory approval for the Edwards Sapien 3 transcatheter ...
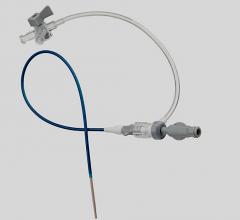
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform ...
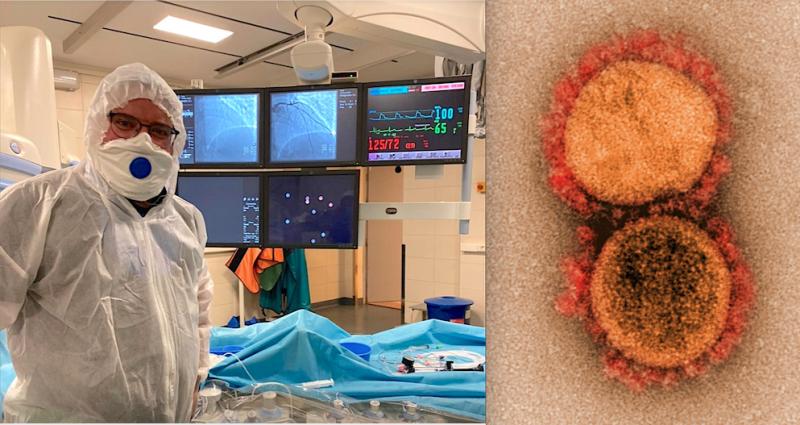
It was originally thought novel coronavirus (COVID-19, SARS-CoV-2) was primarily a respiratory disorder, but as larger ...
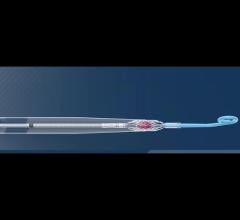
June 5, 2020 — Abiomed announced the U.S. Food and Drug Administration (FDA) has approved the company's investigational ...
This is a quick animation demonstrating how the new 9 French Abiomed Impella ECP expands to approximately 18 French and ...


 June 23, 2020
June 23, 2020