Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new Brainomix 360 Next Generation platform at the International Stroke Conference (ISC) ...
FDA
Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new ...
Feb. 2, 2026 — GE HealthCare has announced that Allia Moveo has received U.S. Food and Drug Administration (FDA) 510(k) ...
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 6, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has announced FDA clearance to expand its ...
Jan. 13, 2026 – Innovative Health, Inc. has received its 50th clearance from FDA to reprocess single-use medical devices ...
Jan. 8, 2026 — AccurKardia recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the ...
Jan. 13, 2026 — AliveCor has received U.S. Food and Drug Administration (FDA) clearance for the next generation of KAI ...
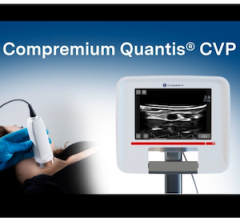
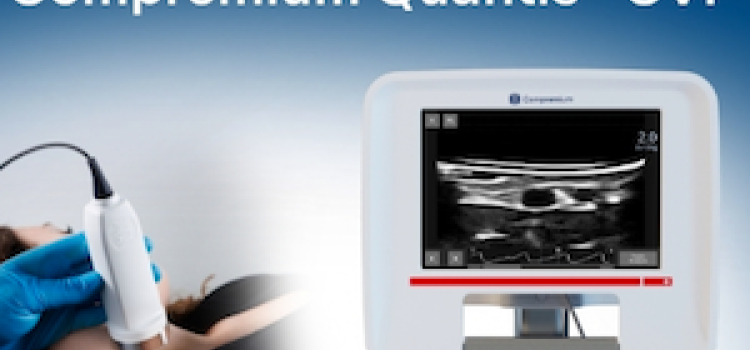
Jan. 8, 2026 — Compremium AG recently announced that the U.S. Food and Drug Administration (FDA) has granted ...
Jan. 12, 2026 — HeartLung Corp. has announced U.S. Food and Drug Administration (FDA) clearance of AI-CVD, its AI ...
Jan. 6, 2026 — W. L. Gore & Associates’ medical business (Gore) has announced the FDA approval of the Gore Viabahn ...
Jan. 6, 2026 — Stereotaxis, a supplier of surgical robotics for minimally invasive endovascular intervention, has ...
Dec. 22, 2025 — Abbott recently announced the U.S. Food and Drug Administration (FDA) has approved the company's Volt ...
Dec. 26, 2025 — Edwards Lifesciences has announced the company’s SAPIEN M3 mitral valve replacement system, a ...
Dec. 18, 2025 — Huxley Medical has announced the SANSA home sleep apnea test has received regulatory clearance from the ...




 February 02, 2026
February 02, 2026