January 29, 2025 – Roche announced today that the Tina-quant Lipoprotein (a) Gen.2 Molarity assay has received 510(k) ...
FDA
Jan. 29, 2025 – Scandinavian Real Heart AB (recently aannounced that its total artificial heart, Realheart TAH, has been ...
Jan. 28, 2025 — Instylla, Inc., a privately held clinical-stage company developing solutions for peripheral vascular ...
Jan. 8, 2025 — Mineralys Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on developing medicines ...
Jan. 7, 2025 — FIRE1 recently announced it has received Breakthrough Device Designation from the U.S. Food and Drug ...
Dec. 12, 2024 — Physicians have a new treatment option for many of the sickest pediatric patients with heart failure and ...
Nov. 22, 2024 — BridgeBio Pharma, Inc. recently announced that the U.S. Food and Drug Administration (FDA) approved ...
Nov, 4, 2024 – R3 Vascular Inc. has announced that the U.S. Food and Drug Administration (FDA) granted investigational ...
Oct. 24, 2024 — Medtronic plc has announced United States Food and Drug Administration (FDA) approval of the Affera ...
Oct. 30, 2024 — HeartLung Technologies, a developer of AI tools for early detection of heart disease, lung cancer and ...
Oct. 18, 2024 — Boston Scientific Corp. recently announced it has received U.S. Food and Drug Administration (FDA) ...
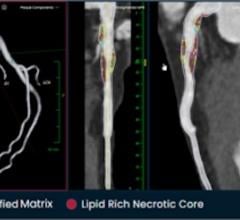
PHOTO CAPTION: The Elucid PlaqueIQ user interface is a fully interactive visualization of the patient’s coronary anatomy ...
Sept. 24, 2024 — Thrombolytic Science, LLC (TSI) has announced that the U.S. Food and Drug Administration (FDA) has ...
Sept. 16, 2024 — Biotronik has received U.S. Food and Drug Administration (FDA) labeling approval of its Selectra 3D ...
Sept. 16, 2024 – Shockwave Medical, Inc., part of Johnson & Johnson MedTech, has announced the full U.S. launch of the ...


 February 03, 2025
February 03, 2025