Aug. 25, 2025 — Amgen has announced that the U.S. Food and Drug Administration (FDA) has broadened the approved use of ...
FDA
Aug. 14, 2025 — During HeartBeam, Inc.'s second quarter 2025 earnings conference call, the medical technology company ...
July 30. 2025 — Cardiosense, a medical AI company, recently announced that the U.S. Food and Drug Administration (FDA) ...
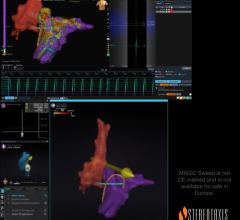
July 28, 2025 — Stereotaxis, a provider of surgical robotics for minimally invasive endovascular intervention, has ...
July 14, 2025 – Johnson & Johnson MedTech has announced U.S. Food and Drug Administration (FDA) approval of an update ...
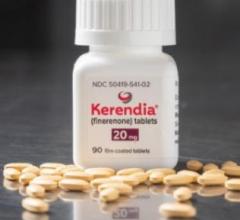
July 14, 2025 — Bayer has announced that the U.S. Food and Drug Administration (FDA) approved KERENDIA (finerenone) to ...
July 10, 2025 — On July 2, 2025, Centers for Medicare & Medicaid Services (CMS) issued a National Coverage Determination ...
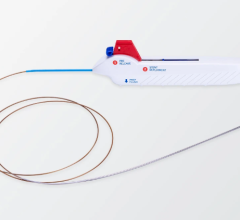
July 9, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...
July 7, 2025 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval to expand the ...
June 04, 2025 — HeartSciences Inc. has announced that the U.S. Food and Drug Administration has granted Breakthrough ...
June 12, 2025 — Viz.ai recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
June 5, 2025 – Penumbra, Inc. has announced the U.S. Food and Drug Administration (FDA) clearance and launch of the Ruby ...
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne ...
May 19, 2025 — Gradient Denervation Technologies recently announced the company’s pulmonary denervation system has ...


 August 26, 2025
August 26, 2025