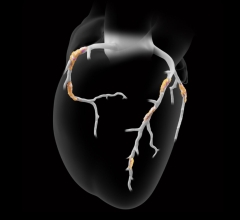
Oct. 14, 2025 — Nurea has announced that its PRAEVAorta 2 software has received FDA 510(k) clearance, enabling entry ...
FDA
Oct. 13, 2025 — Medtronic plc recently announced it has received U.S. Food and Drug Administration (FDA) labeling ...
Oct. 7, 2025 — Nobles Medical Technology II, a provider of cardiovascular closure solutions, has announced that the U.S ...
Oct. 2, 2025 — AorticLab recently announced the U.S. Food and Drug Administration (FDA) has approved its Investigational ...
Sept. 29, 2025 — AngioSafe has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ...
Sept. 22, 2025 — Heartflow, Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Next Gen ...
Sept. 16, 2025 — Conavi Medical Corp., a commercial-stage medical device company focused on designing, manufacturing and ...
Sept. 8, 2025 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Microbot Medical's Liberty ...
Sept. 2, 2025 — GE HealthCare recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for ...
Sept. 3, 2025 — Kardium Inc. recently announced it has received pre-market approval (PMA) for the Globe Pulsed Field ...
Sept. 2, 2025 — Imperative Care, Inc. has announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its ...
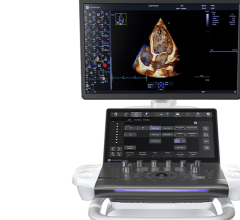
Aug. 29, 2025 — GE HealthCare has launched Vivid Pioneer, its most advanced, ultra-premium and adaptive cardiovascular ...
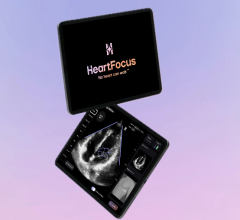
Aug. 27, 2025 – HeartFocus, the AI-enabled cardiac imaging software developed by data-driven medtech company DESKi ...
Aug. 28, 2025 — Medtronic plc has announced it received U.S. Food and Drug Administration (FDA) approval for the ...
Aug. 27, 2025 — Royal Philips has released Transcend Plus, the next generation of its EPIQ CVx and Affiniti CVx ...


 October 14, 2025
October 14, 2025